当前位置:
X-MOL 学术
›
Miner. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Flotation separation of fluorite from calcite by using psyllium seed gum as depressant
Minerals Engineering ( IF 4.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.mineng.2020.106514 Hepeng Zhou , Yongbing Zhang , Xuekun Tang , Yijun Cao , Xianping Luo
Minerals Engineering ( IF 4.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.mineng.2020.106514 Hepeng Zhou , Yongbing Zhang , Xuekun Tang , Yijun Cao , Xianping Luo
|
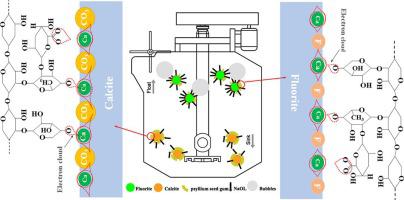
Abstract Separating fluorite and calcite through flotation is difficult due to their similar Ca2+ sites on the adsorption surface of collectors. Psyllium seed gum, a reagent naturally derived from psyllium seed, was used for the first time as an efficient depressant in the flotation separation of fluorite and calcite. Micro-flotation experiments showed that psyllium seed gum had a strong depressing effect on calcite at a wide pH range (8–12). Calcite recovery was significantly and sharply reduced from 97.38% to less than 10% in the presence of psyllium seed gum at pH 9.0. By contrast, fluorite recovery was slightly affected by psyllium seed gum and maintained above 90%. FTIR analysis, XPS analysis, and adsorption test were conducted to determine the selective depression mechanism of psyllium seed gum on these two minerals, and the results show the adsorption effect and capacity of psyllium seed gum were stronger and higher on the calcite surface than on the fluorite surface. Owing to the high electronegativity and powerful electron-gaining ability of CO32−, many electron clouds moved from the hydroxyl groups of psyllium seed gum to the Ca, C, and O elements on the calcite surface. A large amount of psyllium seed gum was chemisorbed on the calcite surface by reacting with Ca2+ site and preventing NaOl adsorption. On the contrary, psyllium seed gum barely affected NaOl adsorption on the fluorite surface. In this case, the efficient flotation separation of fluorite from calcite is realized.
中文翻译:

以车前子胶为抑制剂从方解石中浮选萤石
摘要 萤石和方解石在捕收剂吸附面上具有相似的Ca2+位点,因此很难通过浮选分离萤石和方解石。车前子胶是一种天然衍生自车前子种子的试剂,首次在萤石和方解石的浮选分离中用作有效抑制剂。微浮选实验表明,车前子胶在很宽的 pH 值范围(8-12)下对方解石有很强的抑制作用。在 pH 值为 9.0 的车前子胶存在下,方解石的回收率从 97.38% 显着且急剧地降低至不到 10%。相比之下,萤石回收率受洋车前子胶的影响较小,并保持在 90% 以上。通过FTIR分析、XPS分析和吸附试验,确定了车前子胶对这两种矿物质的选择性抑制机制,结果表明,车前子胶在方解石表面的吸附效果和吸附能力强于萤石表面。由于 CO32− 的高电负性和强大的电子获得能力,许多电子云从车前子胶的羟基转移到方解石表面的 Ca、C 和 O 元素。大量洋车前子胶通过与Ca2+位点反应并阻止NaOl吸附而化学吸附在方解石表面。相反,车前子胶几乎不影响萤石表面对 NaOl 的吸附。在这种情况下,实现了萤石与方解石的高效浮选分离。由于 CO32− 的高电负性和强大的电子获得能力,许多电子云从车前子胶的羟基转移到方解石表面的 Ca、C 和 O 元素。大量洋车前子胶通过与Ca2+位点反应并阻止NaOl吸附而化学吸附在方解石表面。相反,车前子胶几乎不影响萤石表面对 NaOl 的吸附。在这种情况下,实现了萤石与方解石的高效浮选分离。由于 CO32− 的高电负性和强大的电子获得能力,许多电子云从车前子胶的羟基转移到方解石表面的 Ca、C 和 O 元素。大量洋车前子胶通过与Ca2+位点反应并阻止NaOl吸附而化学吸附在方解石表面。相反,车前子胶几乎不影响萤石表面对 NaOl 的吸附。在这种情况下,实现了萤石与方解石的高效浮选分离。洋车前子胶几乎不影响萤石表面的 NaOl 吸附。在这种情况下,实现了萤石与方解石的高效浮选分离。洋车前子胶几乎不影响萤石表面的 NaOl 吸附。在这种情况下,实现了萤石与方解石的高效浮选分离。
更新日期:2020-12-01
中文翻译:

以车前子胶为抑制剂从方解石中浮选萤石
摘要 萤石和方解石在捕收剂吸附面上具有相似的Ca2+位点,因此很难通过浮选分离萤石和方解石。车前子胶是一种天然衍生自车前子种子的试剂,首次在萤石和方解石的浮选分离中用作有效抑制剂。微浮选实验表明,车前子胶在很宽的 pH 值范围(8-12)下对方解石有很强的抑制作用。在 pH 值为 9.0 的车前子胶存在下,方解石的回收率从 97.38% 显着且急剧地降低至不到 10%。相比之下,萤石回收率受洋车前子胶的影响较小,并保持在 90% 以上。通过FTIR分析、XPS分析和吸附试验,确定了车前子胶对这两种矿物质的选择性抑制机制,结果表明,车前子胶在方解石表面的吸附效果和吸附能力强于萤石表面。由于 CO32− 的高电负性和强大的电子获得能力,许多电子云从车前子胶的羟基转移到方解石表面的 Ca、C 和 O 元素。大量洋车前子胶通过与Ca2+位点反应并阻止NaOl吸附而化学吸附在方解石表面。相反,车前子胶几乎不影响萤石表面对 NaOl 的吸附。在这种情况下,实现了萤石与方解石的高效浮选分离。由于 CO32− 的高电负性和强大的电子获得能力,许多电子云从车前子胶的羟基转移到方解石表面的 Ca、C 和 O 元素。大量洋车前子胶通过与Ca2+位点反应并阻止NaOl吸附而化学吸附在方解石表面。相反,车前子胶几乎不影响萤石表面对 NaOl 的吸附。在这种情况下,实现了萤石与方解石的高效浮选分离。由于 CO32− 的高电负性和强大的电子获得能力,许多电子云从车前子胶的羟基转移到方解石表面的 Ca、C 和 O 元素。大量洋车前子胶通过与Ca2+位点反应并阻止NaOl吸附而化学吸附在方解石表面。相反,车前子胶几乎不影响萤石表面对 NaOl 的吸附。在这种情况下,实现了萤石与方解石的高效浮选分离。洋车前子胶几乎不影响萤石表面的 NaOl 吸附。在这种情况下,实现了萤石与方解石的高效浮选分离。洋车前子胶几乎不影响萤石表面的 NaOl 吸附。在这种情况下,实现了萤石与方解石的高效浮选分离。



























 京公网安备 11010802027423号
京公网安备 11010802027423号