Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2020-09-02 , DOI: 10.1016/j.jaci.2020.07.036 Pascal Demoly 1 , Jonathan Corren 2 , Peter Creticos 3 , Frédéric De Blay 4 , Philippe Gevaert 5 , Peter Hellings 6 , Krzysztof Kowal 7 , Martine Le Gall 8 , Natalia Nenasheva 9 , Giovanni Passalacqua 10 , Oliver Pfaar 11 , Miguel Tortajada-Girbés 12 , Carmen Vidal 13 , Margitta Worm 14 , Thomas B Casale 15
|
Background
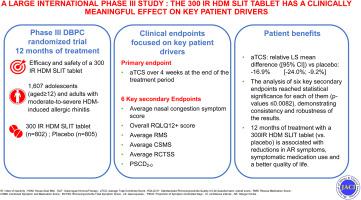
Allergic rhinitis induced by house dust mites (HDMs) is a highly prevalent but often underdiagnosed and undertreated/untreated chronic disease. It often has a negative impact on sleep, work, leisure activities, and health-related quality of life. Allergen immunotherapy is a proven, safe treatment for respiratory allergies.
Objective
We sought to assess the efficacy and safety of a 300 index of reactivity (IR) sublingual tablet formulation of Dermatophagoides pteronyssinus:Dermatophagoides farinae 1:1 extract in adolescents (aged ≥12) and adults with moderate to severe HDM-induced allergic rhinitis.
Methods
In a phase III, international, double-blind, placebo-controlled, randomized clinical trial, participants received approximately 12 months of treatment with placebo or the 300 IR tablet. The primary end point was the average total combined score during 4 weeks at the end of the treatment period.
Results
A total of 1607 participants were randomized, and 1476 (including 555 [37.6%] with concomitant mild controlled asthma at inclusion) comprised the full analysis set. Over the primary evaluation period, the least squares mean average total combined score in the 300 IR group (3.62) was significantly lower (P < .0001) than in the placebo group (4.35), with a relative least squares mean difference of −16.9% (95% CI, −24.0% to −9.2%). All prespecified secondary end points were consistently improved in the 300 IR group, relative to placebo. The 300 IR tablet was generally well tolerated. Treatment-related adverse events (mainly mild or moderate local reactions) were reported for 51.0% of the patients in the 300 IR group and 14.9% in the placebo group.
Conclusions
The 300 IR sublingual HDM tablet is an effective, safe treatment for HDM-induced allergic rhinitis.
中文翻译:

300 IR 舌下含片可有效、安全地治疗屋尘螨引起的过敏性鼻炎:一项国际、双盲、安慰剂对照、随机 III 期临床试验
背景
由屋尘螨 (HDM) 引起的过敏性鼻炎是一种非常普遍但经常未被诊断和治疗/未治疗的慢性疾病。它通常会对睡眠、工作、休闲活动和与健康相关的生活质量产生负面影响。过敏原免疫疗法是一种经过验证的、安全的呼吸道过敏治疗方法。
客观的
我们试图评估 300 指数反应性 (IR) 的屋尘螨舌下片制剂的有效性和安全性:尘螨1:1 提取物在患有中度至重度 HDM 引起的过敏性鼻炎的青少年(≥12 岁)和成人中的有效性和安全性。
方法
在 III 期、国际、双盲、安慰剂对照、随机临床试验中,参与者接受了大约 12 个月的安慰剂或 300 IR 片剂治疗。主要终点是治疗期结束时 4 周内的平均总综合评分。
结果
共有 1607 名参与者被随机分组,其中 1476 名(包括 555 名 [37.6%] 在纳入时伴有轻度控制哮喘)构成了完整的分析集。在主要评估期间,300 IR 组的最小二乘平均总综合得分 (3.62) 显着低于 安慰剂组 (4.35) ( P < .0001),相对最小二乘平均差异为 -16.9 %(95% CI,-24.0% 至 -9.2%)。相对于安慰剂,300 IR 组的所有预设次要终点均持续改善。300 IR 片剂通常耐受性良好。300 IR 组中 51.0% 的患者和安慰剂组中 14.9% 的患者报告了与治疗相关的不良事件(主要是轻度或中度局部反应)。
结论
300 IR 舌下含服 HDM 片剂是治疗 HDM 引起的过敏性鼻炎的有效、安全的治疗方法。










































 京公网安备 11010802027423号
京公网安备 11010802027423号