当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The mechanisms of the formation of surface passivating phases and their impact on the kinetics of galena leaching in ferric chloride, ferric perchlorate, and ferric nitrate solutions
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.hydromet.2020.105468 Fatemeh Nikkhou , Fang Xia , Artur P. Deditius , Xizhi Yao
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.hydromet.2020.105468 Fatemeh Nikkhou , Fang Xia , Artur P. Deditius , Xizhi Yao
|
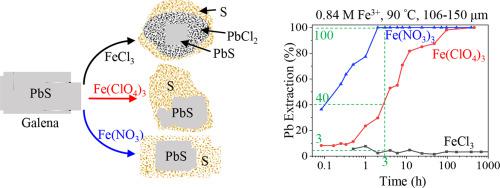
Abstract Hydrometallurgical leaching of Pb from galena is an environmentally friendly alternative to the traditional pyrometallurgical processing. Leaching produces secondary phases passivating the surface of galena and has a negative impact on the leaching rate and Pb extraction, yet a comprehensive understanding of surface passivation is lacking. Here we studied galena leaching in FeCl3, Fe(ClO4)3, and Fe(NO3)3 solutions, using the ex situ leaching-quenching-characterization method complemented by in situ synchrotron powder X-ray diffraction. We examined the effect of salt concentration (0.42 and 0.84 M), temperature (35–90 °C), time (up to 1440 h), solid-weight-to-fluid-volume ratio (0.5–50 g·L−1), and particle size (up to 355 μm) on the mechanisms and kinetics of galena leaching. The results show that leaching in FeCl3 was least effective due to precipitation of a cotunnite (PbCl2) layer on galena surface, and a layer of elemental sulfur on cotunnite, forming a core/mantle/shell structure (i.e., galena/cotunnite/sulfur), which restricted Pb extraction to less than 9%. Sulfur encapsulated PbCl2 and limited its dissolution even when the bulk solution remained undersaturated with respect to PbCl2. Leaching in Fe(ClO4)3 and Fe(NO3)3 solutions was more effective than in FeCl3, achieving 100% Pb extraction. Leaching in Fe(NO3)3 was fastest among the three iron salts. Porous elemental sulfur was the only secondary phase formed using Fe(NO3)3 and Fe(ClO4)3 and the leaching reactions follow the interface-coupled dissolution-reprecipitation mineral replacement mechanism. The effect of passivation was observed when the elemental sulfur layer reached ~20 μm thick. The activation energies calculated by the modified “time-to-a-given-fraction” method were 5.4–67.2 kJ·mol−1 for leaching with Fe(ClO4)3 and 11.4–56.8 kJ·mol−1 with Fe(NO3)3, indicating that leaching was controlled by surface reactions and phase boundary diffusion. This study provides new insights into the mechanisms controlling surface passivation due to the mineralogical and textural compositions of the passivating layers and the resulting leaching kinetics. It provides scientific bases for the development of heap and in situ leaching protocols for Pb extraction from galena ores.
中文翻译:

表面钝化相的形成机制及其对氯化铁、高氯酸铁和硝酸铁溶液中方铅矿浸出动力学的影响
摘要 方铅矿湿法冶金浸出铅是传统火法冶金工艺的一种环保替代方法。浸出产生第二相钝化方铅矿表面,对浸出率和铅提取产生负面影响,但缺乏对表面钝化的全面了解。在这里,我们研究了方铅矿在 FeCl3、Fe(ClO4)3 和 Fe(NO3)3 溶液中的浸出,使用非原位浸出-淬火-表征方法辅以原位同步加速器粉末 X 射线衍射。我们检查了盐浓度(0.42 和 0.84 M)、温度(35–90 °C)、时间(长达 1440 小时)、固重与流体体积比(0.5–50 g·L−1 ) 和粒度(最大 355 μm)对方铅矿浸出的机制和动力学的影响。结果表明,在 FeCl3 中的浸出效果最差,因为方铅矿表面上沉淀了一层铅锌矿 (PbCl2),并且在铅锌矿上形成了一层元素硫,形成了核/地幔/壳结构(即方铅矿/钴镍矿/硫) ,这将 Pb 提取限制在 9% 以下。硫包封 PbCl2 并限制其溶解,即使主体溶液相对于 PbCl2 仍保持不饱和。在 Fe(ClO4)3 和 Fe(NO3)3 溶液中浸出比在 FeCl3 中浸出更有效,可实现 100% 的 Pb 提取。在三种铁盐中,Fe(NO3)3 的浸出速度最快。多孔元素硫是使用 Fe(NO3)3 和 Fe(ClO4)3 形成的唯一次生相,浸出反应遵循界面耦合溶解-再沉淀矿物置换机制。当元素硫层达到~20 μm 厚时,观察到钝化效果。通过改进的“时间到给定分数”方法计算的活化能为 Fe(ClO4)3 浸出为 5.4-67.2 kJ·mol-1 和 Fe(NO3) 浸出为 11.4-56.8 kJ·mol-1 3,表明浸出受表面反应和相界扩散控制。由于钝化层的矿物学和结构组成以及由此产生的浸出动力学,这项研究为控制表面钝化的机制提供了新的见解。它为开发从方铅矿矿石中提取铅的堆浸和原位浸出方案提供了科学依据。8 kJ·mol-1 与 Fe(NO3)3,表明浸出受表面反应和相界扩散控制。由于钝化层的矿物学和结构组成以及由此产生的浸出动力学,这项研究为控制表面钝化的机制提供了新的见解。它为开发从方铅矿矿石中提取铅的堆浸和原位浸出方案提供了科学依据。8 kJ·mol-1 与 Fe(NO3)3,表明浸出受表面反应和相界扩散控制。由于钝化层的矿物学和结构组成以及由此产生的浸出动力学,这项研究为控制表面钝化的机制提供了新的见解。它为开发从方铅矿矿石中提取铅的堆浸和原位浸出方案提供了科学依据。
更新日期:2020-11-01
中文翻译:

表面钝化相的形成机制及其对氯化铁、高氯酸铁和硝酸铁溶液中方铅矿浸出动力学的影响
摘要 方铅矿湿法冶金浸出铅是传统火法冶金工艺的一种环保替代方法。浸出产生第二相钝化方铅矿表面,对浸出率和铅提取产生负面影响,但缺乏对表面钝化的全面了解。在这里,我们研究了方铅矿在 FeCl3、Fe(ClO4)3 和 Fe(NO3)3 溶液中的浸出,使用非原位浸出-淬火-表征方法辅以原位同步加速器粉末 X 射线衍射。我们检查了盐浓度(0.42 和 0.84 M)、温度(35–90 °C)、时间(长达 1440 小时)、固重与流体体积比(0.5–50 g·L−1 ) 和粒度(最大 355 μm)对方铅矿浸出的机制和动力学的影响。结果表明,在 FeCl3 中的浸出效果最差,因为方铅矿表面上沉淀了一层铅锌矿 (PbCl2),并且在铅锌矿上形成了一层元素硫,形成了核/地幔/壳结构(即方铅矿/钴镍矿/硫) ,这将 Pb 提取限制在 9% 以下。硫包封 PbCl2 并限制其溶解,即使主体溶液相对于 PbCl2 仍保持不饱和。在 Fe(ClO4)3 和 Fe(NO3)3 溶液中浸出比在 FeCl3 中浸出更有效,可实现 100% 的 Pb 提取。在三种铁盐中,Fe(NO3)3 的浸出速度最快。多孔元素硫是使用 Fe(NO3)3 和 Fe(ClO4)3 形成的唯一次生相,浸出反应遵循界面耦合溶解-再沉淀矿物置换机制。当元素硫层达到~20 μm 厚时,观察到钝化效果。通过改进的“时间到给定分数”方法计算的活化能为 Fe(ClO4)3 浸出为 5.4-67.2 kJ·mol-1 和 Fe(NO3) 浸出为 11.4-56.8 kJ·mol-1 3,表明浸出受表面反应和相界扩散控制。由于钝化层的矿物学和结构组成以及由此产生的浸出动力学,这项研究为控制表面钝化的机制提供了新的见解。它为开发从方铅矿矿石中提取铅的堆浸和原位浸出方案提供了科学依据。8 kJ·mol-1 与 Fe(NO3)3,表明浸出受表面反应和相界扩散控制。由于钝化层的矿物学和结构组成以及由此产生的浸出动力学,这项研究为控制表面钝化的机制提供了新的见解。它为开发从方铅矿矿石中提取铅的堆浸和原位浸出方案提供了科学依据。8 kJ·mol-1 与 Fe(NO3)3,表明浸出受表面反应和相界扩散控制。由于钝化层的矿物学和结构组成以及由此产生的浸出动力学,这项研究为控制表面钝化的机制提供了新的见解。它为开发从方铅矿矿石中提取铅的堆浸和原位浸出方案提供了科学依据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号