当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Dynamics Simulation of the n-Octacosane-Water Mixture Confined in Hydrophilic and Hydrophobic Mesopores: The Effect of Oxygenates
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.fluid.2020.112816 Konstantinos D. Papavasileiou , Loukas D. Peristeras , Jiaqi Chen , Gerard P. van der Laan , Indranil Rudra , Ahmad Kalantar , Ioannis G. Economou
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.fluid.2020.112816 Konstantinos D. Papavasileiou , Loukas D. Peristeras , Jiaqi Chen , Gerard P. van der Laan , Indranil Rudra , Ahmad Kalantar , Ioannis G. Economou
|
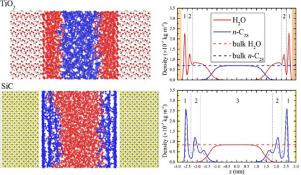
Abstract Wax and water mixtures are the main products of the Gas-To-Liquids (GTL) process through the Fischer-Tropsch synthesis (FTS), along with relatively small quantities of oxygenated compounds such as alcohols. It has been established that water production in high concentrations leads to phase segregation inside the catalyst support pores. Excess water conditions are considered responsible for sintering of catalytic nanoparticles, which reduces catalyst lifetime and increases GTL operational cost. Apart from the effects of increased water concentration in wax – water FTS mixtures, the presence of oxygenates has not been studied at the molecular level. Moreover, the effect of the surface support chemistry on the wax – water mixture inside catalyst pores is essential in understanding mixture phase behavior. All these open questions are of significant interest for the petrochemical industry. The present study focuses on simulating by means of Molecular Dynamics (MD) the n-octacosane (n-C28) – water mixture inside titanium dioxide (TiO2) and silicon carbide (SiC) mesopores at low-temperature FTS conditions (473.15 K). The presence of small alcohols such as methanol and ethanol on the n-C28 – H2O mixture's phase behavior was also evaluated. The united atom TraPPE (TraPPE-UA) force field was used for n-C28 and the alcohols, while the SPC/E was used for water. Catalyst support atoms were modeled as Lennard-Jones spheres (charged or neutral) at fixed positions. Our simulations show that inside TiO2, water molecules organize into two discrete layers on the TiO2 surface, with n-C28 occupying the pore center; mixture phase separation is completely reversed inside the SiC pore. In both cases, alcohol addition does not influence either n-C28 or H2O distribution inside the pores. Furthermore, we examine the hydrogen bonds formed between water molecules and how these are influenced upon alcohol addition and confinement. Finally, through the calculation of lateral diffusion coefficients, we provide insights on the mobility of the individual n-C28 – H2O mixture components inside the pores considered.
中文翻译:

亲水性和疏水性介孔中正二十八烷-水混合物的分子动力学模拟:含氧物的影响
摘要 蜡和水的混合物是通过费托合成 (FTS) 进行的气转液 (GTL) 工艺的主要产品,以及相对少量的含氧化合物,如醇。已经确定,高浓度的水产生导致催化剂载体孔内的相分离。过量的水条件被认为是导致催化纳米颗粒烧结的原因,这会降低催化剂寿命并增加 GTL 运行成本。除了蜡 - 水 FTS 混合物中水浓度增加的影响外,尚未在分子水平上研究含氧化合物的存在。此外,表面载体化学对催化剂孔内蜡-水混合物的影响对于理解混合物相行为至关重要。所有这些悬而未决的问题都对石化行业具有重要意义。本研究的重点是通过分子动力学 (MD) 在低温 FTS 条件 (473.15 K) 下模拟二氧化钛 (TiO2) 和碳化硅 (SiC) 介孔内的正辛烷 (n-C28)-水混合物。还评估了正 C28 – H2O 混合物的相行为中存在的甲醇和乙醇等小醇。联合原子 TraPPE (TraPPE-UA) 力场用于 n-C28 和醇,而 SPC/E 用于水。催化剂载体原子被建模为固定位置的 Lennard-Jones 球体(带电或中性)。我们的模拟表明,在 TiO2 内部,水分子在 TiO2 表面组织成两个离散层,n-C28 占据孔隙中心;SiC 孔内的混合物相分离完全逆转。在这两种情况下,添加醇都不会影响孔内的 n-C28 或 H2O 分布。此外,我们检查了水分子之间形成的氢键以及它们如何影响酒精添加和限制。最后,通过横向扩散系数的计算,我们提供了对所考虑孔内单个 n-C28-H2O 混合物组分的迁移率的见解。
更新日期:2020-12-01
中文翻译:

亲水性和疏水性介孔中正二十八烷-水混合物的分子动力学模拟:含氧物的影响
摘要 蜡和水的混合物是通过费托合成 (FTS) 进行的气转液 (GTL) 工艺的主要产品,以及相对少量的含氧化合物,如醇。已经确定,高浓度的水产生导致催化剂载体孔内的相分离。过量的水条件被认为是导致催化纳米颗粒烧结的原因,这会降低催化剂寿命并增加 GTL 运行成本。除了蜡 - 水 FTS 混合物中水浓度增加的影响外,尚未在分子水平上研究含氧化合物的存在。此外,表面载体化学对催化剂孔内蜡-水混合物的影响对于理解混合物相行为至关重要。所有这些悬而未决的问题都对石化行业具有重要意义。本研究的重点是通过分子动力学 (MD) 在低温 FTS 条件 (473.15 K) 下模拟二氧化钛 (TiO2) 和碳化硅 (SiC) 介孔内的正辛烷 (n-C28)-水混合物。还评估了正 C28 – H2O 混合物的相行为中存在的甲醇和乙醇等小醇。联合原子 TraPPE (TraPPE-UA) 力场用于 n-C28 和醇,而 SPC/E 用于水。催化剂载体原子被建模为固定位置的 Lennard-Jones 球体(带电或中性)。我们的模拟表明,在 TiO2 内部,水分子在 TiO2 表面组织成两个离散层,n-C28 占据孔隙中心;SiC 孔内的混合物相分离完全逆转。在这两种情况下,添加醇都不会影响孔内的 n-C28 或 H2O 分布。此外,我们检查了水分子之间形成的氢键以及它们如何影响酒精添加和限制。最后,通过横向扩散系数的计算,我们提供了对所考虑孔内单个 n-C28-H2O 混合物组分的迁移率的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号