Cell Calcium ( IF 4.3 ) Pub Date : 2020-09-02 , DOI: 10.1016/j.ceca.2020.102284 Miguel A Chiurillo 1 , Noelia Lander 1 , Anibal E Vercesi 2 , Roberto Docampo 3
|
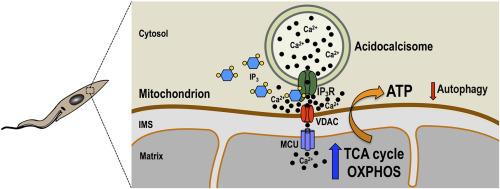
In contrast to animal cells, the inositol 1,4,5-trisphosphate receptor of Trypanosoma cruzi (TcIP3R) localizes to acidocalcisomes instead of the endoplasmic reticulum. Here, we present evidence that TcIP3R is a Ca2+ release channel gated by IP3 when expressed in DT40 cells knockout for all vertebrate IP3 receptors, and is required for Ca2+ uptake by T. cruzi mitochondria, regulating pyruvate dehydrogenase dephosphorylation and mitochondrial O2 consumption, and preventing autophagy. Localization studies revealed its co-localization with an acidocalcisome marker in all life cycle stages of the parasite. Ablation of TcIP3R by CRISPR/Cas9 genome editing caused: a) a reduction in O2 consumption rate and citrate synthase activity; b) decreased mitochondrial Ca2+ transport without affecting the membrane potential; c) increased ammonia production and AMP/ATP ratio; d) stimulation of autophagosome formation, and e) marked defects in growth of culture forms (epimastigotes) and invasion of host cells by infective stages (trypomastigotes). Moreover, TcIP3R overexpressing parasites showed decreased metacyclogenesis, trypomastigote host cell invasion and intracellular amastigote replication. In conclusion, the results suggest a modulatory activity of TcIP3R-mediated acidocalcisome Ca2+ release on cell bioenergetics in T. cruzi.
中文翻译:

IP3 受体介导的酸钙体释放 Ca2+ 调节线粒体生物能量学并防止克氏锥虫的自噬。
与动物细胞相比,克氏锥虫(TcIP 3 R)的肌醇 1,4,5-三磷酸受体定位于酸钙体而不是内质网。在这里,我们提供的证据表明,当 TcIP 3 R在 DT40 细胞敲除所有脊椎动物 IP 3受体时表达时,TcIP 3 R 是一个由 IP 3门控的 Ca 2+释放通道,并且是T. cruzi线粒体摄取Ca 2+所必需的,调节丙酮酸脱氢酶去磷酸化和线粒体 O 2消耗,并防止自噬。定位研究揭示了它在寄生虫的所有生命周期阶段与酸钙体标记物的共定位。CRISPR/Cas9 基因组编辑对 TcIP 3 R 的消融导致:a) O 2消耗率和柠檬酸合酶活性降低;b) 减少线粒体 Ca 2+转运而不影响膜电位;c) 增加氨产量和 AMP/ATP 比率;d) 刺激自噬体形成,和 e) 培养形式(外鞭毛体)的生长和感染阶段(trypomastigotes)侵入宿主细胞的显着缺陷。此外,TcIP 3R 过表达的寄生虫表现出减少的metacyclogenesis、trypomastigote 宿主细胞侵袭和细胞内无鞭毛体复制。总之,结果表明TcIP 3 R 介导的酸钙体Ca 2+释放对T. cruzi细胞生物能量学的调节活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号