Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 3 ) Pub Date : 2020-09-02 , DOI: 10.1016/j.bbagen.2020.129724 Guangyan Zhou 1 , Shidong Chu 1 , Aditya Kohli 2 , Francis C Szoka 3 , Miriam Gochin 4
|
Background
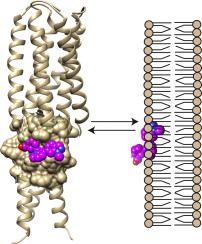
The hydrophobic pocket (HP) of HIV-1 glycoprotein-41 ectodomain is defined by two chains of the N-heptad repeat trimer, within the protein-protein interface that mediates 6HB formation. It is a potential target for inhibitors of viral fusion, but its hydrophobic nature and proximity to membrane in situ has precluded ready analysis of inhibitor interactions.
Methods
We evaluated the sensitivity of 19F NMR and fluorescence for detecting peptide and small molecule binding to the HP and explored the effect of non-denaturing detergent or phospholipid as cosolvents and potential mimics of the membrane environment surrounding gp41.
Results
Chemical shifts of aromatic fluorines were found to be sensitive to changes in the hydrogen bonding network that occurred when inhibitors transitioned from solvent into the HP or into ordered detergent micelles. Fluorescence intensities and emission maxima of autofluorescent compounds responded to changes in the local environment.
Conclusions
Gp41 – ligand binding occurred under all conditions, but was diminished in the presence of detergents. NMR and fluorescence studies revealed that dodecylphosphocholine (DPC) was a poor substitute for membrane in this system, while liposomes could mimic the membrane surroundings.
General significance
Our findings suggest that development of high potency small molecule binders to the HP may be frustrated by competition between binding to the HP and binding to the bilayer membrane.
中文翻译:

HIV-1 糖蛋白 41 与肽和小分子相互作用的生物物理学研究 - 脂质和洗涤剂的影响。
背景
HIV-1 糖蛋白-41 胞外域的疏水口袋 (HP) 由 N-七肽重复三聚体的两条链定义,位于介导 6HB 形成的蛋白质-蛋白质界面内。它是病毒融合抑制剂的潜在靶标,但其疏水性和原位膜的接近性阻碍了对抑制剂相互作用的现成分析。
方法
我们评估了19 F NMR 和荧光检测与 HP 结合的肽和小分子的灵敏度,并探讨了非变性洗涤剂或磷脂作为共溶剂和 gp41 周围膜环境的潜在模拟物的影响。
结果
发现芳族氟的化学位移对当抑制剂从溶剂转变为 HP 或有序洗涤剂胶束时发生的氢键网络变化很敏感。自发荧光化合物的荧光强度和发射最大值对当地环境的变化做出反应。
结论
Gp41 – 配体结合在所有条件下都会发生,但在有去污剂的情况下会减弱。核磁共振和荧光研究表明,十二烷基磷酸胆碱 (DPC) 是该系统中膜的不良替代品,而脂质体可以模拟膜环境。
一般意义
我们的研究结果表明,与 HP 结合和与双层膜结合之间的竞争可能会阻碍对 HP 的高效小分子结合剂的开发。



























 京公网安备 11010802027423号
京公网安备 11010802027423号