Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-09-02 , DOI: 10.1016/j.apsb.2020.08.014 Xiaopan Gao 1 , Bo Qin 1 , Pu Chen 1 , Kaixiang Zhu 1 , Pengjiao Hou 1 , Justyna Aleksandra Wojdyla 2 , Meitian Wang 2 , Sheng Cui 1
|
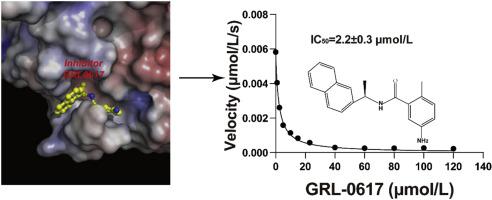
The pandemic of coronavirus disease 2019 (COVID-19) is changing the world like never before. This crisis is unlikely contained in the absence of effective therapeutics or vaccine. The papain-like protease (PLpro) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) plays essential roles in virus replication and immune evasion, presenting a charming drug target. Given the PLpro proteases of SARS-CoV-2 and SARS-CoV share significant homology, inhibitor developed for SARS-CoV PLpro is a promising starting point of therapeutic development. In this study, we sought to provide structural frameworks for PLpro inhibitor design. We determined the unliganded structure of SARS-CoV-2 PLpro mutant C111S, which shares many structural features of SARS-CoV PLpro. This crystal form has unique packing, high solvent content and reasonable resolution 2.5 Å, hence provides a good possibility for fragment-based screening using crystallographic approach. We characterized the protease activity of PLpro in cleaving synthetic peptide harboring nsp2/nsp3 juncture. We demonstrate that a potent SARS-CoV PLpro inhibitor GRL0617 is highly effective in inhibiting protease activity of SARS-CoV-2 with the IC50 of 2.2 ± 0.3 μmol/L. We then determined the structure of SARS-CoV-2 PLpro complexed by GRL0617 to 2.6 Å, showing the inhibitor accommodates the S3–S4 pockets of the substrate binding cleft. The binding of GRL0617 induces closure of the BL2 loop and narrows the substrate binding cleft, whereas the binding of a tetrapeptide substrate enlarges the cleft. Hence, our results suggest a mechanism of GRL0617 inhibition, that GRL0617 not only occupies the substrate pockets, but also seals the entrance to the substrate binding cleft hence prevents the binding of the LXGG motif of the substrate.
中文翻译:

SARS-CoV-2 木瓜蛋白酶样蛋白酶的晶体结构。
2019 年冠状病毒病 (COVID-19) 的大流行正在以前所未有的方式改变世界。如果没有有效的治疗方法或疫苗,这场危机不太可能得到遏制。严重急性呼吸综合征冠状病毒2(SARS-CoV-2)的木瓜蛋白酶(PLpro)在病毒复制和免疫逃避中发挥着重要作用,是一个迷人的药物靶点。鉴于 SARS-CoV-2 和 SARS-CoV 的 PLpro 蛋白酶具有显着的同源性,为 SARS-CoV PLpro 开发的抑制剂是治疗开发的一个有希望的起点。在这项研究中,我们试图为 PLpro 抑制剂设计提供结构框架。我们确定了 SARS-CoV-2 PLpro 突变体 C111S 的无配体结构,它与 SARS-CoV PLpro 具有许多结构特征。该晶型具有独特的堆积、高溶剂含量和合理的2.5 Å分辨率,因此为利用晶体学方法进行基于片段的筛选提供了良好的可能性。我们表征了 PLpro 在裂解含有 nsp2/nsp3 连接处的合成肽时的蛋白酶活性。我们证明了一种有效的 SARS-CoV PLpro 抑制剂 GRL0617 可高度有效地抑制 SARS-CoV-2 的蛋白酶活性,IC 50为 2.2 ± 0.3 μmol/L。然后,我们确定了 GRL0617 与 2.6 Å 复合的 SARS-CoV-2 PLpro 的结构,表明该抑制剂可容纳底物结合裂口的 S3-S4 口袋。GRL0617 的结合诱导 BL2 环闭合并缩小底物结合裂口,而四肽底物的结合则扩大裂口。因此,我们的结果表明GRL0617的抑制机制是GRL0617不仅占据底物口袋,而且密封底物结合裂口的入口,从而阻止底物的LXGG基序的结合。










































 京公网安备 11010802027423号
京公网安备 11010802027423号