当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Origin and stabilization of axial chirality in the construction of naphthyl-C2-indoles: a DFT study
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-09-01 , DOI: 10.1039/d0qo00936a Qianqian Deng 1, 2, 3, 4, 5 , Yang Wang 6, 7, 8, 9 , Shi-Jun Li 1, 2, 3, 4, 5 , Ling-Bo Qu 1, 2, 3, 4, 5 , Yu Lan 1, 2, 3, 4, 5 , Donghui Wei 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-09-01 , DOI: 10.1039/d0qo00936a Qianqian Deng 1, 2, 3, 4, 5 , Yang Wang 6, 7, 8, 9 , Shi-Jun Li 1, 2, 3, 4, 5 , Ling-Bo Qu 1, 2, 3, 4, 5 , Yu Lan 1, 2, 3, 4, 5 , Donghui Wei 1, 2, 3, 4, 5
Affiliation
|
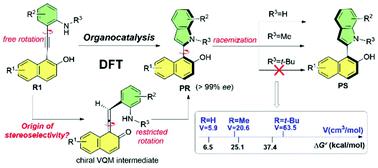
The origin and maintenance of axial chirality in the construction of naphthyl-C2-indoles, via the asymmetric annulation of ortho-alkynylaniline catalyzed by a chiral Brønsted base (cinchonine–thiourea), have been systematically explored using density functional theory (DFT). Several key processes are included in the general mechanism of this kind of reaction: hydrogen bond adsorption of the ortho-alkynylaniline by a catalyst, proton transfer for forming an ortho-quinone methide (VQM) intermediate, intramolecular cyclization, and release of the catalyst to generate R- or S-axial-chiral aryl-C2-indole skeletons. The stereoselectivity-determining steps were identified, and the calculated results show that the pathway associated with the R-configurational isomer has the lower energy barrier, so the corresponding product should be the main one. Non-covalent interaction and atom-in-molecule analyses indicate that N–H⋯O, C–H⋯π and π⋯π interactions play important roles in controlling the stereoselectivity. Multiple processes and different substituents were employed to compute the energy barriers for transformation between R- and S-configurational isomers, revealing that the volume of the substituents is key for maintaining high axial chirality. The obtained insights into the origin for generating and stabilizing the high axial chirality is valuable for the rational design of more efficient organocatalytic reactions.
中文翻译:

萘-C2-吲哚结构的轴向手性的起源和稳定性:DFT研究
通过使用密度泛函理论(DFT)系统地研究了手性Brønsted碱(辛可宁-硫脲)催化邻位炔基苯胺的不对称环化,萘基-C2-吲哚结构的轴向手性的起源和维持。这种反应的一般机理包括几个关键过程:催化剂对邻炔基苯胺的氢键吸附,形成邻醌甲基化物(VQM)中间体的质子转移,分子内环化以及将催化剂释放为产生R-或S-轴向手性芳基-C 2-吲哚骨架。确定了确定立体选择性的步骤,计算结果表明,与R-构型异构体相关的途径具有较低的能垒,因此相应的产物应为主要产物。非共价相互作用和分子内原子分析表明,N–H⋯O,C–H⋯π和π⋯π相互作用在控制立体选择性中起重要作用。使用多个过程和不同的取代基来计算R-和S之间转化的能垒-构型异构体,表明取代基的体积是保持高轴向手性的关键。对于生成和稳定高轴向手性的起源所获得的见解对于合理设计更有效的有机催化反应是有价值的。
更新日期:2020-10-13
中文翻译:

萘-C2-吲哚结构的轴向手性的起源和稳定性:DFT研究
通过使用密度泛函理论(DFT)系统地研究了手性Brønsted碱(辛可宁-硫脲)催化邻位炔基苯胺的不对称环化,萘基-C2-吲哚结构的轴向手性的起源和维持。这种反应的一般机理包括几个关键过程:催化剂对邻炔基苯胺的氢键吸附,形成邻醌甲基化物(VQM)中间体的质子转移,分子内环化以及将催化剂释放为产生R-或S-轴向手性芳基-C 2-吲哚骨架。确定了确定立体选择性的步骤,计算结果表明,与R-构型异构体相关的途径具有较低的能垒,因此相应的产物应为主要产物。非共价相互作用和分子内原子分析表明,N–H⋯O,C–H⋯π和π⋯π相互作用在控制立体选择性中起重要作用。使用多个过程和不同的取代基来计算R-和S之间转化的能垒-构型异构体,表明取代基的体积是保持高轴向手性的关键。对于生成和稳定高轴向手性的起源所获得的见解对于合理设计更有效的有机催化反应是有价值的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号