当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Orbital energy mismatch engenders high-spin ground states in heterobimetallic complexes
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-01 , DOI: 10.1039/d0sc03777j Scott C. Coste 1, 2, 3, 4 , Tyler J. Pearson 1, 2, 3, 4 , Alison B. Altman 1, 2, 3, 4 , Ryan A. Klein 1, 2, 3, 4 , Brian A. Finney 1, 4, 5, 6 , Michael Y. Hu 4, 7, 8, 9 , E. Ercan Alp 4, 7, 8, 9 , Bess Vlaisavljevich 1, 4, 5, 6 , Danna E. Freedman 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-01 , DOI: 10.1039/d0sc03777j Scott C. Coste 1, 2, 3, 4 , Tyler J. Pearson 1, 2, 3, 4 , Alison B. Altman 1, 2, 3, 4 , Ryan A. Klein 1, 2, 3, 4 , Brian A. Finney 1, 4, 5, 6 , Michael Y. Hu 4, 7, 8, 9 , E. Ercan Alp 4, 7, 8, 9 , Bess Vlaisavljevich 1, 4, 5, 6 , Danna E. Freedman 1, 2, 3, 4
Affiliation
|
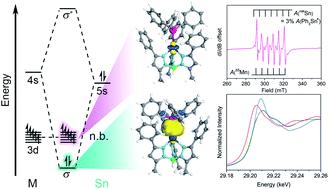
The spin state in heterobimetallic complexes heavily influences both reactivity and magnetism. Exerting control over spin states in main group-based heterobimetallics requires a different approach as the orbital interactions can differ substantially from that of classic coordination complexes. By deliberately engendering an energetic mismatch within the two metals in a bimetallic complex we can mimic the electronic structure of lanthanides. Towards this end, we report a new family of complexes, [Ph,MeTpMSnPh3] where M = Mn (3), Fe (4), Co (5), Ni (6), Zn (7), featuring unsupported bonding between a transition metal and Sn which represent an unusual high spin electronic structure. Analysis of the frontier orbitals reveal the desired orbital mismatch with Sn 5s/5p primarily interacting with 4s/4p M orbitals yielding localized, non-bonding d orbitals. This approach offers a mechanism to design and control spin states in bimetallic complexes.
中文翻译:

轨道能量失配导致异双金属配合物中的高自旋基态
异双金属配合物中的自旋状态严重影响反应性和磁性。对基于主族的异双金属化合物的自旋态进行控制需要采用不同的方法,因为轨道相互作用可能与经典配位化合物大不相同。通过在双金属络合物中故意在两种金属之间产生能量失配,我们可以模拟镧系元素的电子结构。为此,我们报告了一个新的配合物家族[ Ph,Me TpMSnPh 3 ],其中M = Mn(3),Fe(4),Co(5),Ni(6),Zn(7),其特征在于过渡金属和Sn之间不受支撑的结合,这代表了一种不寻常的高自旋电子结构。对前沿轨道的分析表明,与Sn 5s / 5p的轨道失配主要与4s / 4p M轨道相互作用,从而产生局部的非键合d轨道。这种方法提供了一种设计和控制双金属配合物中自旋态的机制。
更新日期:2020-09-23
中文翻译:

轨道能量失配导致异双金属配合物中的高自旋基态
异双金属配合物中的自旋状态严重影响反应性和磁性。对基于主族的异双金属化合物的自旋态进行控制需要采用不同的方法,因为轨道相互作用可能与经典配位化合物大不相同。通过在双金属络合物中故意在两种金属之间产生能量失配,我们可以模拟镧系元素的电子结构。为此,我们报告了一个新的配合物家族[ Ph,Me TpMSnPh 3 ],其中M = Mn(3),Fe(4),Co(5),Ni(6),Zn(7),其特征在于过渡金属和Sn之间不受支撑的结合,这代表了一种不寻常的高自旋电子结构。对前沿轨道的分析表明,与Sn 5s / 5p的轨道失配主要与4s / 4p M轨道相互作用,从而产生局部的非键合d轨道。这种方法提供了一种设计和控制双金属配合物中自旋态的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号