当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction Mechanism of Anionic Lignin and Cationic Soft Surface in Saline Systems.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.jpcb.0c04442 Niloofar Alipoormazandarani 1 , Pedram Fatehi 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.jpcb.0c04442 Niloofar Alipoormazandarani 1 , Pedram Fatehi 1
Affiliation
|
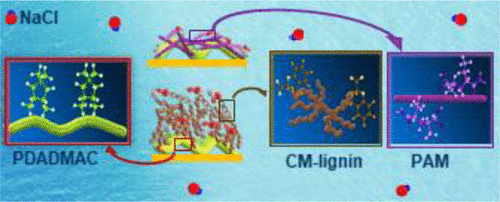
Lignin has a complicated three-dimensional structure that is different from other synthetic and bio-based materials. In this work, we first examined the physicochemical behavior, i.e., apparent hydrodynamic radius (Rh) and ζ-potential, of carboxymethylated lignin (CM) in a saline system. Then, the detailed interaction and adsorption behavior of CM on a cationic poly(diallyldimethylammonium chloride) (PDADMAC)-coated surface were investigated in a saline system by a quartz crystal microbalance with dissipation. The theoretical and experimental adsorption data revealed that CM made limited surface coverage at a low salt concentration via charge neutralization following an intrinsic compensation mechanism. At a higher salt concentration, the adsorption of CM was improved significantly following the extrinsic compensation mechanism and nonionic interaction (e.g., hydrophobic interaction). The adsorption affinity of CM in the urea environment revealed the contribution (10–30%) of hydrogen bonding in the adsorption of CM on the PDADMAC surface. Contrary to what was found for the CM, the adsorption of a linear poly(acrylic acid–acrylamide) (PAM) on the PDADMAC surface exhibited a dramatic decrease at higher salinity, possibly due to the absence of nonionic and hydrophobic interactions between PAM and the surface. The findings of this study showed the superior adsorption performance of the lignin-based polyelectrolytes to the synthetic ones in salt-containing systems.
中文翻译:

盐体系中阴离子木质素与阳离子软表面的相互作用机理。
木质素具有复杂的三维结构,不同于其他合成和生物基材料。在这项工作中,我们首先检查了物理化学行为,即表观流体动力学半径(R h盐溶液中羧甲基化木质素(CM)的ζ电位和ζ电位。然后,在盐体系中,通过耗散的石英晶体微量天平,研究了在阳离子聚二烯丙基二甲基氯化铵(PDADMAC)涂层表面上CM的详细相互作用和吸附行为。理论和实验吸附数据表明,在固有的补偿机制下,通过电荷中和,CM在低盐浓度下的表面覆盖范围有限。在较高的盐浓度下,通过外在补偿机制和非离子相互作用(例如疏水相互作用),CM的吸附显着改善。CM在尿素环境中的吸附亲和力揭示了氢键对PDADMAC表面上CM吸附的贡献(10-30%)。与CM的发现相反,在较高的盐度下,PDADMAC表面线性聚(丙烯酸-丙烯酰胺)(PAM)的吸附量显着降低,这可能是由于PAM与聚合物之间不存在非离子和疏水相互作用所致。表面。这项研究的结果表明,木质素基聚电解质在含盐体系中具有优于合成电解质的吸附性能。
更新日期:2020-10-02
中文翻译:

盐体系中阴离子木质素与阳离子软表面的相互作用机理。
木质素具有复杂的三维结构,不同于其他合成和生物基材料。在这项工作中,我们首先检查了物理化学行为,即表观流体动力学半径(R h盐溶液中羧甲基化木质素(CM)的ζ电位和ζ电位。然后,在盐体系中,通过耗散的石英晶体微量天平,研究了在阳离子聚二烯丙基二甲基氯化铵(PDADMAC)涂层表面上CM的详细相互作用和吸附行为。理论和实验吸附数据表明,在固有的补偿机制下,通过电荷中和,CM在低盐浓度下的表面覆盖范围有限。在较高的盐浓度下,通过外在补偿机制和非离子相互作用(例如疏水相互作用),CM的吸附显着改善。CM在尿素环境中的吸附亲和力揭示了氢键对PDADMAC表面上CM吸附的贡献(10-30%)。与CM的发现相反,在较高的盐度下,PDADMAC表面线性聚(丙烯酸-丙烯酰胺)(PAM)的吸附量显着降低,这可能是由于PAM与聚合物之间不存在非离子和疏水相互作用所致。表面。这项研究的结果表明,木质素基聚电解质在含盐体系中具有优于合成电解质的吸附性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号