当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiconfigurational Calculations and Nonadiabatic Molecular Dynamics Explain Tricyclooctadiene Photochemical Chemoselectivity.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.jpca.0c05280 Jingbai Li 1 , Steven A Lopez 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.jpca.0c05280 Jingbai Li 1 , Steven A Lopez 1
Affiliation
|
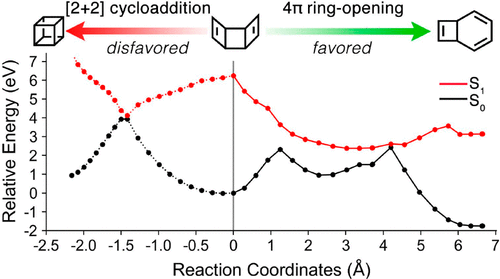
Sunlight is a renewable energy source that can be stored in chemical bonds using photochemical reactions. The synthesis of exotic and strained molecules is especially attractive with photochemical techniques because of the associated efficient and mild reaction conditions. We have understood the photophysics and subsequent photochemistry of a possible cubane precursor, tricyclo[4,2,0,02,5]octa-3,7-diene with complete active space self-consistent field (CASSCF) calculations with an (8,7) active space and the ANO-S-VDZP basis set to. The CASSCF energies were corrected with a second-order perturbative correction CASPT2(8,7)/ANO-S-VDZP. The S0 → S1 vertical excitation energy of 1 is 6.25 eV, which is a π → π* excitation. The minimum energy path from the S1 Franck–Condon point leads to a 4π-disrotatory electrocyclic ring-opening reaction to afford bicyclo[4,2,0]octa-2,4,7-triene. The 2D potential energy surface scan located a rhomboidal S1/S0 minimum energy crossing point connecting 1 and cubane, suggesting that a cycloaddition is theoretically possible. We used the fewest switches surface hopping to study the photodynamics of this cycloaddition: 85% of 1722 trajectories relaxed to eight products; the major products are bicyclo[4,2,0]octa-2,4,7-triene (30%) and cycloocta-1,3,5,7-tetraene (32%). Only 0.4% of trajectories undergo a [2 + 2] cycloaddition to form cubane.
中文翻译:

多构型计算和非绝热分子动力学解释了三环辛二烯的光化学化学选择性。
阳光是一种可再生能源,可以通过光化学反应以化学键形式存储。由于相关的有效和温和的反应条件,奇异分子和应变分子的合成在光化学技术中特别有吸引力。我们已经了解了可能的古巴前体三环[4,2,0,0 2,5 ] octa -3,7-二烯的光物理性质和随后的光化学性质,其中完整的活性空间自洽场(CASSCF)计算为(8 ,7)将活动空间和ANO-S-VDZP的基础设置为。用二阶微扰校正CASPT2(8,7)/ ANO-S-VDZP校正了CASSCF能量。S 0 →S 1的垂直激发能为1是6.25 eV,这是π→π*激发。从S 1 Franck–Condon点的最小能量路径导致4π-可旋转的电环开环反应,生成双环[4,2,0] octa-2,4,7-三烯。二维势能表面扫描位于连接1和古巴的菱形S 1 / S 0最小能量交叉点,这表明理论上可以进行环加成。我们使用最少的开关表面跳变研究了这种环加成反应的光动力学:1722个轨迹中的85%松弛为8个乘积;主要产品是双环[4,2,0] octa-2,4,7-三烯(30%)和环辛-1,3,5,7-四烯(32%)。只有0.4%的轨迹经历了[2 + 2]环加成反应,从而形成了古巴。
更新日期:2020-09-24
中文翻译:

多构型计算和非绝热分子动力学解释了三环辛二烯的光化学化学选择性。
阳光是一种可再生能源,可以通过光化学反应以化学键形式存储。由于相关的有效和温和的反应条件,奇异分子和应变分子的合成在光化学技术中特别有吸引力。我们已经了解了可能的古巴前体三环[4,2,0,0 2,5 ] octa -3,7-二烯的光物理性质和随后的光化学性质,其中完整的活性空间自洽场(CASSCF)计算为(8 ,7)将活动空间和ANO-S-VDZP的基础设置为。用二阶微扰校正CASPT2(8,7)/ ANO-S-VDZP校正了CASSCF能量。S 0 →S 1的垂直激发能为1是6.25 eV,这是π→π*激发。从S 1 Franck–Condon点的最小能量路径导致4π-可旋转的电环开环反应,生成双环[4,2,0] octa-2,4,7-三烯。二维势能表面扫描位于连接1和古巴的菱形S 1 / S 0最小能量交叉点,这表明理论上可以进行环加成。我们使用最少的开关表面跳变研究了这种环加成反应的光动力学:1722个轨迹中的85%松弛为8个乘积;主要产品是双环[4,2,0] octa-2,4,7-三烯(30%)和环辛-1,3,5,7-四烯(32%)。只有0.4%的轨迹经历了[2 + 2]环加成反应,从而形成了古巴。











































 京公网安备 11010802027423号
京公网安备 11010802027423号