当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
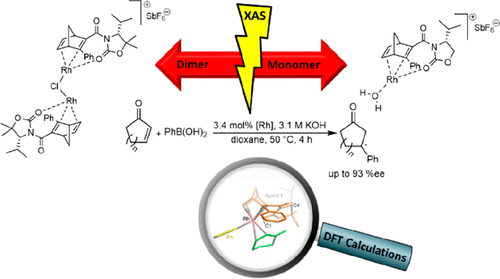
Experimental and Theoretical Study on the Role of Monomeric vs Dimeric Rhodium Oxazolidinone Norbornadiene Complexes in Catalytic Asymmetric 1,2- and 1,4-Additions
Organometallics ( IF 2.8 ) Pub Date : 2020-09-01 , DOI: 10.1021/acs.organomet.0c00310 Manuel Kirchhof 1 , Katrin Gugeler 2 , Felix Richard Fischer 1 , Michal Nowakowski 3 , Alina Bauer 1 , Sonia Alvarez-Barcia 2 , Karina Abitaev 4 , Marc Schnierle 5 , Yaseen Qawasmi 4 , Wolfgang Frey 1 , Angelika Baro 1 , Deven P. Estes 6 , Thomas Sottmann 4 , Mark R. Ringenberg 5 , Bernd Plietker 7 , Matthias Bauer 3 , Johannes Kästner 2 , Sabine Laschat 1
Organometallics ( IF 2.8 ) Pub Date : 2020-09-01 , DOI: 10.1021/acs.organomet.0c00310 Manuel Kirchhof 1 , Katrin Gugeler 2 , Felix Richard Fischer 1 , Michal Nowakowski 3 , Alina Bauer 1 , Sonia Alvarez-Barcia 2 , Karina Abitaev 4 , Marc Schnierle 5 , Yaseen Qawasmi 4 , Wolfgang Frey 1 , Angelika Baro 1 , Deven P. Estes 6 , Thomas Sottmann 4 , Mark R. Ringenberg 5 , Bernd Plietker 7 , Matthias Bauer 3 , Johannes Kästner 2 , Sabine Laschat 1
Affiliation
|
The influence of nuclearity and charge of chiral Rh diene complexes on the activity and enantioselectivity in catalytic asymmetric 1,2-additions of organoboron reagents to N-tosylimines and 1,4-additions to enones was investigated. For this purpose, cationic dimeric Rh(I) complex [(Rh(1))2Cl]SbF6 and cationic monomeric Rh(I) complex [RhOH2(2)]SbF6 were synthesized from oxazolidinone-substituted 3-phenylnorbornadiene ligands 1 and 2, which differ in the substitution pattern at oxazolidinone C-5′ (CMe2 vs CH2) and compared with the corresponding neutral dimeric and monomeric Rh(I) complexes [RhCl(1)]2 and [RhCl(2)]. Structural, electronic, and mechanistic insights were gained by X-ray crystallography, cyclic voltammetry (CV), X-ray absorption spectroscopy (XAS), and DFT calculations. CV revealed an increased stability of cationic vs neutral Rh complexes toward oxidation. Comparison of solid-state and solution XAS (extended X-ray absorption fine structure (EXAFS), X-ray absorption near edge structure (XANES)) data showed that the monomeric Rh complex [RhCl(2)] maintained its electronic state and coordination sphere in solution, whereas the dimeric Rh complex [RhCl(1)]2 exchanges bridging chloro ligands by dioxane molecules in solution. In both 1,2- and 1,4-addition reactions, monomeric Rh complexes [RhCl(2)] and [RhOH2(2)]SbF6 gave better yields as compared to dimeric complexes [RhCl(1)]2 and [(Rh(1))2Cl]SbF6. Regarding enantioselectivities, dimeric Rh species [RhCl(1)]2 and [(Rh(1))2Cl]SbF6 performed better than monomeric Rh species in the 1,2-addition, while the opposite was true for the 1,4-addition. Neutral Rh complexes performed better than cationic complexes. Microemulsions improved the yields of 1,2-additions due to a most probable enrichment of Rh complexes in the amphiphilic film and provided a strong influence of the complex nuclearity and charge on the stereocontrol. A strong nonlinear-like effect (NLLE) was observed in 1,2-additions, when diastereomeric mixtures of ligands 1 and epi-1 were employed. The pronounced substrate dependency of the 1,4-addition could be rationalized by DFT calculations.
中文翻译:

单体与二元恶唑烷酮铑降冰片二烯配合物在催化不对称1,2-和1,4-加成中的作用的实验和理论研究
研究了手性Rh二烯配合物的核化和电荷对有机硼试剂向N-甲苯胺的催化不对称1,2-加成反应和对烯酮的1,4-加成反应的活性和对映选择性的影响。为此,由恶唑烷酮取代的3-苯基降冰片二烯配体合成了阳离子二聚Rh(I)络合物[(Rh(1))2 Cl] SbF 6和阳离子单体Rh(I)络合物[RhOH 2(2)] SbF 6 1和2,在恶唑烷酮C-5'处的取代方式不同(CMe 2与CH 2)并与相应的中性二聚体和单体Rh(I)配合物[RhCl(1)] 2和[RhCl(2)]进行比较。通过X射线晶体学,循环伏安法(CV),X射线吸收光谱法(XAS)和DFT计算获得了结构,电子和机械方面的见识。CV显示阳离子与中性Rh配合物对氧化的稳定性提高。固态和溶液XAS(扩展X射线吸收精细结构(EXAFS),X射线吸收近边缘结构(XANES))数据的比较表明,单体Rh配合物[RhCl(2)]保持其电子状态和配位溶液中的球形,而二聚体Rh复合物[RhCl(1)] 2通过溶液中的二恶烷分子交换架桥氯配体。在1,2-和1,4-加成反应中,单体Rh复合物[RhCl(2)]和[RhOH 2(2)] SbF 6与二聚体复合物[RhCl(1)] 2和[ (Rh(1))2 Cl] SbF 6。关于对映选择性,二聚Rh种类[RhCl(1)] 2和[(Rh(1))2 Cl] SbF 6在1,2-加成中,其性能优于单体Rh物种,而在1,4-加成中则相反。中性Rh络合物的性能优于阳离子络合物。由于两亲膜中最可能的Rh配合物富集,微乳液提高了1,2-加成的收率,并提供了复杂的核和电荷对立体控制的强烈影响。当使用配体1和epi - 1的非对映异构混合物时,在1,2-加成中观察到强烈的类非线性效应(NLLE)。1,4-加成的明显的底物依赖性可以通过DFT计算来合理化。
更新日期:2020-09-14
中文翻译:

单体与二元恶唑烷酮铑降冰片二烯配合物在催化不对称1,2-和1,4-加成中的作用的实验和理论研究
研究了手性Rh二烯配合物的核化和电荷对有机硼试剂向N-甲苯胺的催化不对称1,2-加成反应和对烯酮的1,4-加成反应的活性和对映选择性的影响。为此,由恶唑烷酮取代的3-苯基降冰片二烯配体合成了阳离子二聚Rh(I)络合物[(Rh(1))2 Cl] SbF 6和阳离子单体Rh(I)络合物[RhOH 2(2)] SbF 6 1和2,在恶唑烷酮C-5'处的取代方式不同(CMe 2与CH 2)并与相应的中性二聚体和单体Rh(I)配合物[RhCl(1)] 2和[RhCl(2)]进行比较。通过X射线晶体学,循环伏安法(CV),X射线吸收光谱法(XAS)和DFT计算获得了结构,电子和机械方面的见识。CV显示阳离子与中性Rh配合物对氧化的稳定性提高。固态和溶液XAS(扩展X射线吸收精细结构(EXAFS),X射线吸收近边缘结构(XANES))数据的比较表明,单体Rh配合物[RhCl(2)]保持其电子状态和配位溶液中的球形,而二聚体Rh复合物[RhCl(1)] 2通过溶液中的二恶烷分子交换架桥氯配体。在1,2-和1,4-加成反应中,单体Rh复合物[RhCl(2)]和[RhOH 2(2)] SbF 6与二聚体复合物[RhCl(1)] 2和[ (Rh(1))2 Cl] SbF 6。关于对映选择性,二聚Rh种类[RhCl(1)] 2和[(Rh(1))2 Cl] SbF 6在1,2-加成中,其性能优于单体Rh物种,而在1,4-加成中则相反。中性Rh络合物的性能优于阳离子络合物。由于两亲膜中最可能的Rh配合物富集,微乳液提高了1,2-加成的收率,并提供了复杂的核和电荷对立体控制的强烈影响。当使用配体1和epi - 1的非对映异构混合物时,在1,2-加成中观察到强烈的类非线性效应(NLLE)。1,4-加成的明显的底物依赖性可以通过DFT计算来合理化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号