当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium-Catalyzed Asymmetric Hydrogenation of α-Fluoro Ketones via a Dynamic Kinetic Resolution Strategy.
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.orglett.0c02565 Xuefeng Tan 1 , Weijun Zeng 1 , Jialin Wen 1, 2 , Xumu Zhang 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.orglett.0c02565 Xuefeng Tan 1 , Weijun Zeng 1 , Jialin Wen 1, 2 , Xumu Zhang 1
Affiliation
|
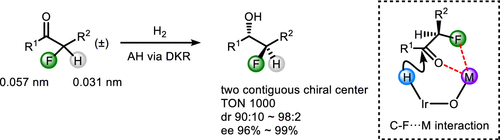
The discrimination of a fluorine atom from a hydrogen atom has been challenging in asymmetric catalysis. We herein report iridium-catalyzed hydrogenation of α-fluoro ketones using a strategy of dynamic kinetic resolution. Both enantiomeric and diastereomeric selectivities were satisfactory in the preparation of β-fluoro alcohols. The DFT calculation revealed a C–F···Na charge–dipole interaction in the transition state of hydride transfer. This noncovalent interaction would be responsible for the diastereomeric control.
中文翻译:

通过动态动力学拆分策略,铱催化α-氟代酮的不对称加氢。
在不对称催化中,将氟原子与氢原子区分开是具有挑战性的。我们在此报告使用动态动力学拆分策略的铱催化的α-氟代酮氢化。在制备β-氟代醇中,对映异构体和非对映异构体的选择性均令人满意。DFT计算表明,在氢化物转移的过渡态中存在CF-·Na电荷-偶极相互作用。这种非共价相互作用将负责非对映异构体的控制。
更新日期:2020-09-20
中文翻译:

通过动态动力学拆分策略,铱催化α-氟代酮的不对称加氢。
在不对称催化中,将氟原子与氢原子区分开是具有挑战性的。我们在此报告使用动态动力学拆分策略的铱催化的α-氟代酮氢化。在制备β-氟代醇中,对映异构体和非对映异构体的选择性均令人满意。DFT计算表明,在氢化物转移的过渡态中存在CF-·Na电荷-偶极相互作用。这种非共价相互作用将负责非对映异构体的控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号