当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exceptionally High O-H Bond Dissociation Free Energy of a Dicopper(II) µ-Hydroxo Complex and Insights into the Geometric and Electronic Structure Origins Thereof
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-01 , DOI: 10.1021/jacs.0c06425 Peter E VanNatta 1 , David A Ramirez 1 , Andres R Velarde 1 , Ghazanfar Ali 1 , Matthew T Kieber-Emmons 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-01 , DOI: 10.1021/jacs.0c06425 Peter E VanNatta 1 , David A Ramirez 1 , Andres R Velarde 1 , Ghazanfar Ali 1 , Matthew T Kieber-Emmons 1
Affiliation
|
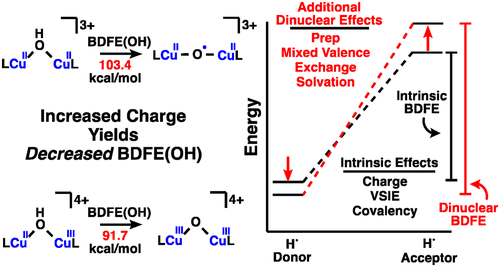
The strength of the relevant bonds in bond-making and bond-breaking processes can directly affect the overall efficiency of the process. Copper-oxygen sites are known to catalyze reactions with some of the most recalcitrant C-H bonds found in nature as quantified by bond dissociation free energy (BDFE), yet only a handful of copper-bound O-H bond strengths have been defined. Equally important in the design of synthetic catalysts is an understanding of the geometric and electronic structure origins of these thermodynamic parameters. In this report, the BDFE(OH) of two dicopper-hydroxo complexes, {[LCu]2-(µ-OH)}3+ and {[LCu]2-(µ-OH)}4+ (L = tris(2-pyridylmethyl)amine), were measured. Two key observations were made: i) the BDFE(OH) of these complexes were exceptionally high at 103.4 kcal/mol and 91.7 kcal/mol respectively, which are the highest condensed phase MO-H BDFE to date; and ii) that the higher oxidation state had a lower BDFE(OH), which is counter to expectations based on known mononuclear BDFE(OH) which increase with oxidation state. To understand the origin of these thermodynamic values, BDFE(OH) were measured and analyzed for the mononuclear complexes [LCu(OH2)]1+ and [LCu(OH2)]2+ in the same ligand environment. This treatment revealed "dinuclear effects" that include contributions from rehybridization of the oxygen, mixed-valency of the metals, magnetic exchange between the metals, and differences in solvation, and which are general with respect to [M]2-OH complexes to varying degrees. These analyses are important because they provide a starting point for rationally tuning the thermodynamics of catalytic intermediates broadly and for understanding how copper active-sites achieve activation of strong C-H bonds.
中文翻译:

Dicopper (II) µ-Hydroxo 络合物的超高 OH 键解离自由能及其几何和电子结构起源的见解
键合和断键过程中相关键的强度可以直接影响过程的整体效率。已知铜氧位点催化与自然界中发现的一些最顽固的 CH 键反应,如键解离自由能 (BDFE) 所量化,但只有少数铜键合 OH 键强度已被定义。在合成催化剂的设计中同样重要的是理解这些热力学参数的几何和电子结构起源。在本报告中,两种二铜-羟基配合物 {[LCu]2-(µ-OH)}3+ 和 {[LCu]2-(µ-OH)}4+ 的 BDFE(OH) (L = tris( 2-吡啶基甲基)胺),进行了测量。进行了两个关键观察:i) 这些配合物的 BDFE(OH) 异常高,分别为 103.4 kcal/mol 和 91.7 kcal/mol,这是迄今为止最高的凝聚相 MO-H BDFE;ii) 较高的氧化态具有较低的 BDFE(OH),这与基于已知单核 BDFE(OH) 的预期相反,后者随着氧化态的增加而增加。为了了解这些热力学值的来源,测量并分析了 BDFE(OH) 在相同配体环境中的单核配合物 [LCu(OH2)]1+ 和 [LCu(OH2)]2+。这种处理揭示了“双核效应”,包括氧的再杂化、金属的混合价态、金属之间的磁交换和溶剂化的差异,这些作用对于 [M]2-OH 复合物来说是普遍的度。
更新日期:2020-09-01
中文翻译:

Dicopper (II) µ-Hydroxo 络合物的超高 OH 键解离自由能及其几何和电子结构起源的见解
键合和断键过程中相关键的强度可以直接影响过程的整体效率。已知铜氧位点催化与自然界中发现的一些最顽固的 CH 键反应,如键解离自由能 (BDFE) 所量化,但只有少数铜键合 OH 键强度已被定义。在合成催化剂的设计中同样重要的是理解这些热力学参数的几何和电子结构起源。在本报告中,两种二铜-羟基配合物 {[LCu]2-(µ-OH)}3+ 和 {[LCu]2-(µ-OH)}4+ 的 BDFE(OH) (L = tris( 2-吡啶基甲基)胺),进行了测量。进行了两个关键观察:i) 这些配合物的 BDFE(OH) 异常高,分别为 103.4 kcal/mol 和 91.7 kcal/mol,这是迄今为止最高的凝聚相 MO-H BDFE;ii) 较高的氧化态具有较低的 BDFE(OH),这与基于已知单核 BDFE(OH) 的预期相反,后者随着氧化态的增加而增加。为了了解这些热力学值的来源,测量并分析了 BDFE(OH) 在相同配体环境中的单核配合物 [LCu(OH2)]1+ 和 [LCu(OH2)]2+。这种处理揭示了“双核效应”,包括氧的再杂化、金属的混合价态、金属之间的磁交换和溶剂化的差异,这些作用对于 [M]2-OH 复合物来说是普遍的度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号