当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Ubiquitin Variants with Selectivity for Ubiquitin C-Terminal Hydrolase Deubiquitinases.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.biochem.9b01076 Chad S Hewitt 1 , Aaron D Krabill 1 , Chittaranjan Das 2, 3 , Daniel P Flaherty 1, 3, 4
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.biochem.9b01076 Chad S Hewitt 1 , Aaron D Krabill 1 , Chittaranjan Das 2, 3 , Daniel P Flaherty 1, 3, 4
Affiliation
|
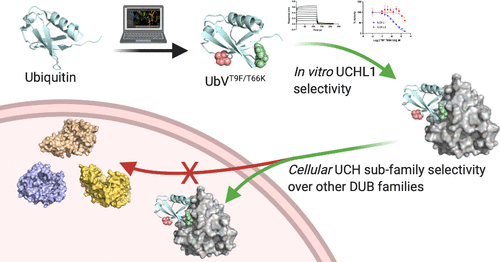
Ubiquitin (Ub) is a highly conserved protein that is covalently attached to substrate proteins as a post-translational modification to regulate signaling pathways such as proteasomal degradation and cell cycle/transcriptional regulation in the eukaryotic cellular environment. Ub signaling is regulated by the homeostasis of substrate protein ubiquitination/deubiquitination by E3 ligases and deubiquitinating enzymes (DUBs) in healthy eukaryotic systems. One such DUB, ubiquitin C-terminal hydrolase L1 (UCHL1), is endogenously expressed in the central nervous system under normal physiological conditions, but overexpression and/or mutation has been linked to various cancers and neurodegenerative diseases. The lack of UCHL1 probing strategies suggests development of a selective Ub variant (UbV) for probing UCHL1’s role in these disease states would be beneficial. We describe a computational design approach to investigate UbVs that lend selectivity, both binding and inhibition, to UCHL1 over the close structural homologue UCHL3 and members of other DUB families. A number of UbVs, mainly those containing Thr9 mutations, displayed appreciable binding and inhibition selectivity for UCHL1 over UCHL3, compared to wild-type Ub in in vitro assays. By appending reactive electrophiles to the C-terminus of the UbVs, we created the first activity-based probe (ABP) with demonstrated reaction selectivity for UCH family DUBs over other families in cell lysates. Further kinetic analysis of covalent inhibition by the UbV-ABP with UCHL1 and UCHL3 offers insight into the future design of UCHL1 selective UbV-ABP. These studies serve as a proof of concept of the viability of the in silico design of ubiquitin variants for UCH family DUBs as a step toward the development of macromolecular UCHL1 inhibitors.
中文翻译:

具有泛素C末端水解酶去泛素酶选择性的泛素变体的开发。
泛素(Ubquitin)(Ub)是一种高度保守的蛋白质,它作为翻译后修饰共价附于底物蛋白质,以调节信号通路,如蛋白酶体降解和真核细胞环境中的细胞周期/转录调控。在健康的真核系统中,Ub信号受底物蛋白泛素化/去泛素化的稳态调节,该平衡由E3连接酶和去泛素化酶(DUB)进行。一种这样的DUB,泛素C末端水解酶L1(UCHL1)在正常生理条件下在中枢神经系统内源性表达,但过表达和/或突变与多种癌症和神经退行性疾病有关。缺乏UCHL1探测策略表明,开发选择性Ub变体(UbV)来探测UCHL1在这些疾病状态中的作用将是有益的。我们描述了一种计算设计方法来研究UbV,这些UbV通过紧密的结构同源物UCHL3和其他DUB家族的成员对UCHL1产生选择性,结合和抑制作用。与野生型Ub相比,许多UbV(主要是那些含有Thr9突变的UbV)对UCHL1的抑制作用相对于UCHL3表现出明显的结合和抑制选择性。体外测定。通过将反应性亲电试剂附加到UbVs的C末端,我们创建了第一个基于活性的探针(ABP),该探针对UCH家族DUB的反应选择性高于细胞裂解液中的其他家族。UbV-ABP与UCHL1和UCHL3对共价抑制的进一步动力学分析,为UCHL1选择性UbV-ABP的未来设计提供了见识。这些研究证明了UCH家族DUB的泛素变体计算机设计的可行性的概念验证,这是朝着开发大分子UCHL1抑制剂迈出的一步。
更新日期:2020-09-22
中文翻译:

具有泛素C末端水解酶去泛素酶选择性的泛素变体的开发。
泛素(Ubquitin)(Ub)是一种高度保守的蛋白质,它作为翻译后修饰共价附于底物蛋白质,以调节信号通路,如蛋白酶体降解和真核细胞环境中的细胞周期/转录调控。在健康的真核系统中,Ub信号受底物蛋白泛素化/去泛素化的稳态调节,该平衡由E3连接酶和去泛素化酶(DUB)进行。一种这样的DUB,泛素C末端水解酶L1(UCHL1)在正常生理条件下在中枢神经系统内源性表达,但过表达和/或突变与多种癌症和神经退行性疾病有关。缺乏UCHL1探测策略表明,开发选择性Ub变体(UbV)来探测UCHL1在这些疾病状态中的作用将是有益的。我们描述了一种计算设计方法来研究UbV,这些UbV通过紧密的结构同源物UCHL3和其他DUB家族的成员对UCHL1产生选择性,结合和抑制作用。与野生型Ub相比,许多UbV(主要是那些含有Thr9突变的UbV)对UCHL1的抑制作用相对于UCHL3表现出明显的结合和抑制选择性。体外测定。通过将反应性亲电试剂附加到UbVs的C末端,我们创建了第一个基于活性的探针(ABP),该探针对UCH家族DUB的反应选择性高于细胞裂解液中的其他家族。UbV-ABP与UCHL1和UCHL3对共价抑制的进一步动力学分析,为UCHL1选择性UbV-ABP的未来设计提供了见识。这些研究证明了UCH家族DUB的泛素变体计算机设计的可行性的概念验证,这是朝着开发大分子UCHL1抑制剂迈出的一步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号