Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Headless C1q: a new molecular tool to decipher its collagen‐like functions
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-08-31 , DOI: 10.1111/febs.15543 Guillaume Fouët 1 , Isabelle Bally 1 , Luca Signor 1 , Katharina Häußermann 2 , Nicole M Thielens 1 , Véronique Rossi 1 , Christine Gaboriaud 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-08-31 , DOI: 10.1111/febs.15543 Guillaume Fouët 1 , Isabelle Bally 1 , Luca Signor 1 , Katharina Häußermann 2 , Nicole M Thielens 1 , Véronique Rossi 1 , Christine Gaboriaud 1
Affiliation
|
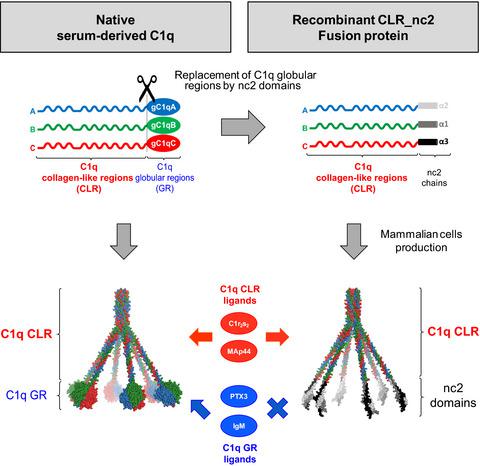
Complement component C1q, a soluble defense collagen, is the recognition protein of the classical complement pathway. C1q is able to recognize and interact with multiple targets and, via the subsequent activation of its cognate serine proteases C1r and C1s, initiates the complement cascade. C1q is made up of six ABC heterotrimers each containing two different functional regions, an N‐terminal collagen‐like region (CLR) and a C‐terminal globular region (GR). These heterotrimers assemble via their N‐terminal regions, resulting in the characteristic ‘bouquet‐like’ shape of C1q with an N‐terminal bundle of collagen fibers with six diverging stems each exhibiting a C‐terminal globular head. The GRs are responsible for the versatile recognition of multiple C1q targets, whereas the CLRs trigger immune response through interacting with several cellular or soluble partners. We report here the generation of the first recombinant form of human C1q without its recognition globular heads. The noncollagenous domain 2 (nc2) of type IX collagen has been substituted for the C1q GR in order to control the correct registering of the collagen triple helices of C1q chains A, B, and C. The resulting CLR_nc2 recombinant protein produced in stably transfected EXPI293 mammalian cells was correctly assembled and folded, as demonstrated by mass spectrometry, mass photometry, and electron microscopy experiments. Its interaction properties were investigated using surface plasmon resonance analysis with known CLR ligands: the tetramer of C1r and C1s dimers and MBL‐associated protein MAp44. Comparison with the interaction properties of native serum‐derived C1q and CLR revealed that recombinant CLR_nc2 retains C1q CLR functional properties.
中文翻译:

无头C1q:破译其胶原样功能的新型分子工具
补体成分C1q(一种可溶性防御胶原蛋白)是经典补体途径的识别蛋白。C1q能够识别多个靶标并与之相互作用,并通过随后激活其关联的丝氨酸蛋白酶C1r和C1s来启动补体级联反应。C1q由六个ABC异源三聚体组成,每个异源三聚体均包含两个不同的功能区,一个N端胶原样区(CLR)和一个C端球状区(GR)。这些异源三聚体通过其N末端区域组装,形成具有C1q特征的“花束状”形状,其中N末端的胶原纤维束带有六个散开的茎,每个茎均具有C末端的球状头。GR负责多种C1q目标的通用识别,而CLR通过与几种细胞或可溶性伴侣相互作用而触发免疫反应。我们在这里报告的人类C1q的第一个重组形式的生成,没有它的识别球状头。IX型胶原蛋白的非胶原结构域2(nc2)已被C1q GR取代,以控制C1q链A,B和C的胶原蛋白三螺旋的正确配准。稳定转染的EXPI293中产生的CLR_nc2重组蛋白质谱,质谱和电子显微镜实验证明,哺乳动物细胞已正确组装和折叠。使用已知的CLR配体:C1r和C1s二聚体的四聚体以及MBL相关蛋白MAp44,通过表面等离振子共振分析研究了其相互作用特性。
更新日期:2020-08-31
中文翻译:

无头C1q:破译其胶原样功能的新型分子工具
补体成分C1q(一种可溶性防御胶原蛋白)是经典补体途径的识别蛋白。C1q能够识别多个靶标并与之相互作用,并通过随后激活其关联的丝氨酸蛋白酶C1r和C1s来启动补体级联反应。C1q由六个ABC异源三聚体组成,每个异源三聚体均包含两个不同的功能区,一个N端胶原样区(CLR)和一个C端球状区(GR)。这些异源三聚体通过其N末端区域组装,形成具有C1q特征的“花束状”形状,其中N末端的胶原纤维束带有六个散开的茎,每个茎均具有C末端的球状头。GR负责多种C1q目标的通用识别,而CLR通过与几种细胞或可溶性伴侣相互作用而触发免疫反应。我们在这里报告的人类C1q的第一个重组形式的生成,没有它的识别球状头。IX型胶原蛋白的非胶原结构域2(nc2)已被C1q GR取代,以控制C1q链A,B和C的胶原蛋白三螺旋的正确配准。稳定转染的EXPI293中产生的CLR_nc2重组蛋白质谱,质谱和电子显微镜实验证明,哺乳动物细胞已正确组装和折叠。使用已知的CLR配体:C1r和C1s二聚体的四聚体以及MBL相关蛋白MAp44,通过表面等离振子共振分析研究了其相互作用特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号