当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cs+/Alcohol Promoted[4C+2C]Annulation: ASynthetic Strategy for Polysubstituted Phenols
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-09-01 , DOI: 10.1002/ajoc.202000435 Xiaohui Yang 1 , Baihui Zheng 1 , Yanqing Wang 1 , Yifei Li 1 , Qun Liu 1 , Ling Pan 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-09-01 , DOI: 10.1002/ajoc.202000435 Xiaohui Yang 1 , Baihui Zheng 1 , Yanqing Wang 1 , Yifei Li 1 , Qun Liu 1 , Ling Pan 1
Affiliation
|
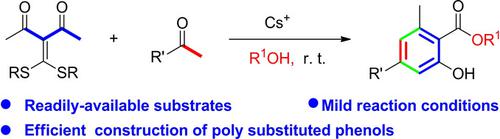
A cesium ion/alcohol promoted [4C+2C]annulation with the easily available diacetyl ketene dithioacetals as 4 C components to give poly‐substituted phenols is reported. It is revealed for the first time that in the presence of cesium ion and alcohol under mild alkaline conditions, the acetyl methyl carbon of ketene dithioacetals can be utilized as a carbon nucleophile. This nucleophile can attack at a carbonyl carbon of a ketone to form a C−C bond to initiate a [4C+2C]annulations, which lead to a poly‐substituted phenol product via intramolecular cyclization followed by aromatization. This reaction provides a new and practical route to the construction of poly‐substituted phenols between the same (two diacetyl ketene dithioacetals) or different ketones (a diacetyl ketene dithioacetal and a ketone) under very mild reaction conditions.
中文翻译:

Cs + /酒精促进的[4C + 2C]环解:多取代苯酚的合成策略
据报道,铯离子/醇促进了[4C + 2C]环化反应,该环化反应以容易获得的二乙酰基乙烯酮二硫缩醛为4 C成分,从而得到多取代的苯酚。首次揭示在铯离子和醇的存在下,在弱碱性条件下,烯酮二硫缩醛的乙酰甲基碳可以用作碳亲核试剂。该亲核试剂可攻击酮的羰基碳以形成C-C键,从而引发[4C + 2C]环化反应,该环化反应通过分子内环化和芳构化作用形成多取代苯酚产物。该反应为在非常温和的反应条件下,在相同的(两个二乙酰基乙烯酮二硫缩醛)或不同的酮类(二乙酰基乙烯酮二硫缩醛和酮)之间构建多取代的酚提供了一条新的实用途径。
更新日期:2020-09-01
中文翻译:

Cs + /酒精促进的[4C + 2C]环解:多取代苯酚的合成策略
据报道,铯离子/醇促进了[4C + 2C]环化反应,该环化反应以容易获得的二乙酰基乙烯酮二硫缩醛为4 C成分,从而得到多取代的苯酚。首次揭示在铯离子和醇的存在下,在弱碱性条件下,烯酮二硫缩醛的乙酰甲基碳可以用作碳亲核试剂。该亲核试剂可攻击酮的羰基碳以形成C-C键,从而引发[4C + 2C]环化反应,该环化反应通过分子内环化和芳构化作用形成多取代苯酚产物。该反应为在非常温和的反应条件下,在相同的(两个二乙酰基乙烯酮二硫缩醛)或不同的酮类(二乙酰基乙烯酮二硫缩醛和酮)之间构建多取代的酚提供了一条新的实用途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号