Structure ( IF 4.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.str.2020.08.006 Dorothy D Majewski 1 , Mark Okon 2 , Florian Heinkel 2 , Craig S Robb 1 , Marija Vuckovic 1 , Lawrence P McIntosh 2 , Natalie C J Strynadka 1
|
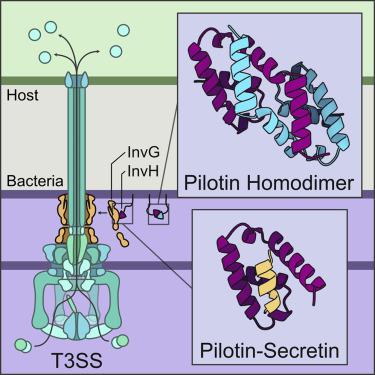
The type III secretion system (T3SS) is a multi-membrane-spanning protein channel used by Gram-negative pathogenic bacteria to secrete effectors directly into the host cell cytoplasm. In the many species reliant on the T3SS for pathogenicity, proper assembly of the outer membrane secretin pore depends on a diverse family of lipoproteins called pilotins. We present structural and biochemical data on the Salmonella enterica pilotin InvH and the S domain of its cognate secretin InvG. Characterization of InvH by X-ray crystallography revealed a dimerized, α-helical pilotin. Size-exclusion-coupled multi-angle light scattering and small-angle X-ray scattering provide supporting evidence for the formation of an InvH homodimer in solution. Structures of the InvH-InvG heterodimeric complex determined by X-ray crystallography and NMR spectroscopy indicate a predominantly hydrophobic interface. Knowledge of the interaction between InvH and InvG brings us closer to understanding the mechanisms by which pilotins assemble the secretin pore.
中文翻译:

使用混合结构方法表征来自肠沙门氏菌 III 型分泌系统的 Pilotin-Secretin 复合物
III 型分泌系统 (T3SS) 是一种跨膜蛋白通道,由革兰氏阴性病原菌用于将效应物直接分泌到宿主细胞质中。在许多依赖 T3SS 致病性的物种中,外膜分泌素孔的正确组装取决于称为引导素的不同脂蛋白家族。我们提供了肠道沙门氏菌的结构和生化数据Pilotin InvH 及其同源分泌素 InvG 的 S 结构域。通过 X 射线晶体学表征 InvH 揭示了二聚化的 α-螺旋引导蛋白。尺寸排阻耦合多角度光散射和小角度 X 射线散射为在溶液中形成 InvH 同源二聚体提供了支持证据。通过 X 射线晶体学和 NMR 光谱确定的 InvH-InvG 异二聚体复合物的结构表明主要是疏水界面。对 InvH 和 InvG 之间相互作用的了解使我们更接近于理解pilotins 组装促胰液素孔的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号