Reactive & Functional Polymers ( IF 4.5 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.reactfunctpolym.2020.104718 Najmeh Parvin , Aziz Babapoor , Ali Nematollahzadeh , Seyyed Mojtaba Mousavi
|
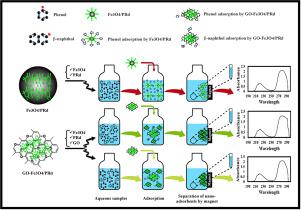
This research focuses on the batch adsorption efficiency of magnetic adsorbents for removal both for phenol and β-naphthol from aqueous solution. Two kinds of nano-adsorbents were investigated: magnetic/polyrhodanine (Fe3O4/PRd) and graphene oxide-magnetic/polyrhodanine (GO-Fe3O4/PRd). GO can improve to be the best nano-adsorbent for phenol and β-naphthol removal due to its large specific surface areas with a maximum adsorption capacity of 191.0 and 226.2 mg g−1, respectively, at contact time 15 min. The characterization of prepared nano-adsorbents by XRD, FESEM, FTIR, VSM, EDS, and BET analysis. The kinetic models indicated that the adsorption process followed a Pseudo-Second-Order model, and the Freundlich and Langmuir isotherm models described the adsorption behavior of phenol and β-naphthol by Fe3O4/PRd and GO-Fe3O4/PRd nano-adsorbents, respectively. Also, thermodynamic studies for both nano-absorbents showed that the absorbing process is an endothermic, spontaneous, and desirable reaction.
中文翻译:

修饰的氧化铁修饰的磁性氧化石墨烯修饰纳米金红花复合材料从水溶液中去除苯酚和β-萘酚的动力学,平衡和热力学研究
这项研究的重点是磁性吸附剂对水溶液中苯酚和β-萘酚的去除效率。研究了两种纳米吸附剂:磁性/聚罗丹宁(Fe 3 O 4 / PRd)和氧化石墨烯-磁性/聚罗丹宁(GO-Fe 3 O 4 / PRd)。GO的比表面积大,最大吸附容量为191.0和226.2 mg g -1,可提高其成为去除苯酚和β-萘酚的最佳纳米吸附剂。,分别在15分钟的联系时间。通过XRD,FESEM,FTIR,VSM,EDS和BET分析对制备的纳米吸附剂进行表征。动力学模型表明吸附过程遵循伪二阶模型,Freundlich和Langmuir等温模型描述了Fe 3 O 4 / PRd和GO-Fe 3 O 4 / PRd对苯酚和β-萘酚的吸附行为。纳米吸附剂。同样,对两种纳米吸收剂的热力学研究表明,吸收过程是吸热的,自发的和理想的反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号