Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.jorganchem.2020.121500 Leandro de O. Amaral , Viner Sousa Lima , Sérgio Macêdo Soares , Julia Bornhorst , Sebastião S. Lemos , Claudia Cristina Gatto , Robert A. Burrow , Priscila Gubert
|
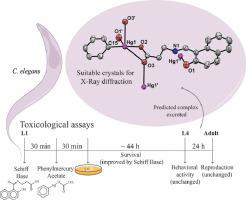
Two series of neutral mercury(II) complexes have been obtained from the reaction of HgPh(OCOCH3)(1, 2, and 3) or Hg(OCOCH3)2(4, 5, and 6) with N-(2-hydroxynaphtalidene)β-alanine (L1H2), N-(5-bromo-salicylidene)β-alanine (L2H2), and N-(5-bromo-salicylidene)β-phenylalanine (L3H2), respectively. The complexes were characterized by elemental analysis, IR, NMR (1H, 13C, and 199Hg) and X-ray crystallography (1 and 2). The elementary analysis showed that the obtained compounds have a high degree of purity and the infrared spectra indicated the shifts of the main functional groups (carboxylate, azomethine and phenol) of the Schiff bases when compared to the respective mercury complexes. X-ray diffraction analyses are similar for complexes 1 and 2, consistent with mononuclear compounds bearing a primarily di-coordinated HgII nucleus with several additional Hg—O interactions, which give rise to supramolecular assemblies. Solution NMR analyses indicate that the primary coordination is retained in DMSO for 1, 2 and 3, but the additional interactions observed in solid state are lost in solution. Complexes 4, 5 and 6 are also mononuclear and all analyses, in both solution and solid state, point to a di-coordinated HgII nucleus with a di-anionic Schiff base ligand. The biological evaluation of the Schiff base (L1H2) chelating potential was performed by pretreating Caenorhabditis elegans (C. elegans) nematodes with the L1H2 (1 mM) followed by phenylmercury acetate exposure (LC50 = 290 μM). The pretreatment with L1H2 resulted in a significant protection of worm survival against phenylmercury acetate toxicity. Together, these data show that the chelating potential of these ligands is a crucial factor in reducing the toxic effects caused by metals in the worm, suggesting them as promising agents in chelation therapy.
中文翻译:

秀丽隐杆线虫中汞(II)与氨基酸衍生的席夫碱配合物的螯合电位的合成,结构表征和评价
HgPh(OCOCH 3)(1、2和3)或Hg(OCOCH 3)2(4、5和6)与N-(2-分别是羟基萘(萘)β-丙氨酸(L1H 2),N-(5-溴-水杨基)β-丙氨酸(L2H 2)和N-(5-溴-水杨基)β-苯丙氨酸(L3H 2)。通过元素分析,IR,NMR(1 H,13 C和199 Hg)和X射线晶体学(1和2)对复合物进行表征。)。元素分析表明,所获得的化合物具有较高的纯度,红外光谱表明,与各汞配合物相比,席夫碱的主要官能团(羧酸根,偶氮甲碱和苯酚)的位移。配合物1和2的X射线衍射分析相似,这与单核化合物带有主要为双配位的Hg II核以及几个其他的Hg-O相互作用是一致的,这导致了超分子组装。溶液NMR分析表明,在1、2和3的DMSO中保留了主要配位,但在溶液中失去了固态观察到的其他相互作用。配合物4,5图6和图6也是单核,并且在溶液和固体状态下的所有分析均指向具有双阴离子席夫碱配体的双配位的Hg II核。通过用L1H 2(1 mM)预处理秀丽隐杆线虫(C. elegans)线虫,然后暴露于乙酸苯汞(LC 50 = 290μM),对席夫碱(L1H 2)螯合电位进行生物学评估。L1H 2预处理从而对蠕虫的存活提供了重要保护,使其免受乙酸苯汞的毒性。总之,这些数据表明,这些配体的螯合潜力是减少蠕虫中金属引起的毒性作用的关键因素,表明它们是螯合疗法中有希望的药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号