当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel homogeneous selective electrocatalysts for CO2 reduction: an electrochemical and computational study of cyclopentadienyl-phenylendiamino-cobalt complexes
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2020-08-31 , DOI: 10.1039/d0se00790k Nicola Melis 1, 2, 3, 4 , Francesca Mocci 2, 4, 5, 6 , Annalisa Vacca 1, 2, 3, 4 , Luca Pilia 1, 2, 3, 4
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2020-08-31 , DOI: 10.1039/d0se00790k Nicola Melis 1, 2, 3, 4 , Francesca Mocci 2, 4, 5, 6 , Annalisa Vacca 1, 2, 3, 4 , Luca Pilia 1, 2, 3, 4
Affiliation
|
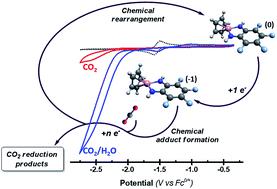
Four cyclopentadienyl-phenylendiamino-cobalt complexes [CoCp(bqdi)] with different substituents (R) at the phenylene moiety (bqdi, I; o-perfluoro-bqdi, II; p-NO2-bqdi, III; p-COOH-bqdi, IV) have been studied with an aim to investigate their capability as catalysts for the CO2 reduction. These compounds were characterized by cyclic voltammetry measurements both under nitrogen and CO2 atmospheres, showing an increase in the cathodic current ranging from 3.36 (III) to 5.59 times (II) that of the measurement under nitrogen. Moreover, with the addition of water, the current enhancement in the presence of CO2 reaches 31.07 times that of the case of complex II. Interestingly, these complexes exhibit very good selectivity toward CO2 reduction irrespective of hydrogen even in the presence of water. The relative turnover frequencies were also estimated, given the values ranging from 3.23 (III) to 187.21 s−1 (II) in the presence of water. In addition, these results were analysed by means of density functional theory (DFT) calculations and Fukui functions analysis. In particular, DFT results clearly show effects of different substituents on the electrochemical properties of these compounds. Whereas, the Fukui functions analysis indicates that the most favourable positions for an electrophilic attack on the reduced complex are the nitrogen and cobalt atoms.
中文翻译:

用于减少二氧化碳的新型均相选择性电催化剂:环戊二烯基-苯基苯二氨基-钴配合物的电化学和计算研究
四个在亚苯基部分具有不同取代基(R)的环戊二烯基-苯基苯二氨基-钴络合物[CoCp(bqdi)] (bqdi,I ;邻-全氟-bqdi,II ; p -NO 2 -bqdi,III ; p -COOH-bqdi ,IV)已经被研究,以研究其作为还原CO 2的催化剂的能力。这些化合物通过在氮气和CO 2气氛下的循环伏安法测量进行表征,显示出的阴极电流增加了3.36(III)至5.59倍(II)在氮气下的测量值。此外,在添加水的情况下,在存在CO 2的情况下,当前的增强作用达到配合物II情况的31.07倍。有趣的是,即使在水的存在下,这些配合物也表现出对CO 2还原的非常好的选择性,而与氢无关。给定值在3.23(III)至187.21 s -1(II)在水的存在下。此外,这些结果还通过密度泛函理论(DFT)计算和Fukui函数分析进行了分析。特别地,DFT结果清楚地显示了不同取代基对这些化合物的电化学性质的影响。而Fukui函数分析表明,对还原的络合物进行亲电攻击的最有利位置是氮和钴原子。
更新日期:2020-09-16
中文翻译:

用于减少二氧化碳的新型均相选择性电催化剂:环戊二烯基-苯基苯二氨基-钴配合物的电化学和计算研究
四个在亚苯基部分具有不同取代基(R)的环戊二烯基-苯基苯二氨基-钴络合物[CoCp(bqdi)] (bqdi,I ;邻-全氟-bqdi,II ; p -NO 2 -bqdi,III ; p -COOH-bqdi ,IV)已经被研究,以研究其作为还原CO 2的催化剂的能力。这些化合物通过在氮气和CO 2气氛下的循环伏安法测量进行表征,显示出的阴极电流增加了3.36(III)至5.59倍(II)在氮气下的测量值。此外,在添加水的情况下,在存在CO 2的情况下,当前的增强作用达到配合物II情况的31.07倍。有趣的是,即使在水的存在下,这些配合物也表现出对CO 2还原的非常好的选择性,而与氢无关。给定值在3.23(III)至187.21 s -1(II)在水的存在下。此外,这些结果还通过密度泛函理论(DFT)计算和Fukui函数分析进行了分析。特别地,DFT结果清楚地显示了不同取代基对这些化合物的电化学性质的影响。而Fukui函数分析表明,对还原的络合物进行亲电攻击的最有利位置是氮和钴原子。









































 京公网安备 11010802027423号
京公网安备 11010802027423号