当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A unique Si-doped carbon nanocatalyst for peroxymonosulfate (PMS) activation: insights into the singlet oxygen generation mechanism and the abnormal salt effect
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-08-31 , DOI: 10.1039/d0en00848f Weijian Duan 1, 2, 3, 4, 5 , Jinglei He 1, 2, 3, 4, 5 , Ziliang Wei 1, 2, 3, 4, 5 , Zongren Dai 1, 2, 3, 4, 5 , Chunhua Feng 1, 2, 3, 4, 5
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-08-31 , DOI: 10.1039/d0en00848f Weijian Duan 1, 2, 3, 4, 5 , Jinglei He 1, 2, 3, 4, 5 , Ziliang Wei 1, 2, 3, 4, 5 , Zongren Dai 1, 2, 3, 4, 5 , Chunhua Feng 1, 2, 3, 4, 5
Affiliation
|
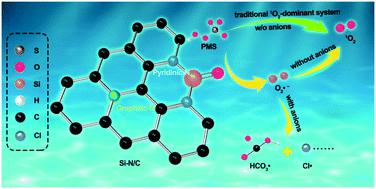
The heteroatom-doped, carbon-activated peroxymonosulfate (PMS) system proceeding via the non-radical oxidation pathway involving singlet oxygen (1O2) represents a promising advanced oxidation process (AOP) due to the resistance of 1O2 to background anions in solution. However, the performance level of pollutant removal is not yet satisfied, and the mechanism of 1O2 generation remains elusive. Herein, we report the development for the first time of a unique Si-engaged heteroatom (i.e., N)-doped carbon (Si/N–C) nanomaterial for PMS activation, which exhibits outstanding performance in the removal of various kinds of pollutants and an appreciably wide working pH range of 2.5 to 11. Taking rhodamine B (RhB) as an example, the Si/N–C@PMS system achieved a much higher reaction rate (0.64 min−1) than the Si–C (0.047 min−1), N–C (0.017 min−1), SiO2 (0.021 min−1), and Co3O4 (0.026 min−1; a standard catalyst) systems. In addition, the results of electron spin resonance (ESR) and scavenging experiments solidly support the conclusion that the mechanism of 1O2 generation in this system is via the recombination of superoxide radicals O2˙−, a pathway seldom reported for metal-free, catalyst-activated PMS systems. More importantly, we observed apparent inhibition effects of the anions (e.g., HCO3−, HPO42−, and PO43−) on the degradation performance, inconsistent with many previous reports suggesting that the 1O2-mediated oxidative system possesses a high selectivity toward pollutant degradation in the presence of these anions. Further studies with the anions, including Cl−, HCO3−, and HxPO4(3−x) (x = 0, 1, 2), suggest that the reaction between O2˙− and these anions is the main reason for the abnormal salt effect. This work will deepen the understanding of 1O2 formation via the intermediate of O2˙− and provide a new insight into its salt resistance capacity.
中文翻译:

独特的硅掺杂的碳纳米催化剂,用于过氧单硫酸盐(PMS)活化:洞察单线态氧生成机理和异常盐效应
通过涉及单线态氧(1 O 2)的非自由基氧化途径进行的杂原子掺杂碳活化过氧单硫酸盐(PMS)系统代表了一种有前途的高级氧化过程(AOP),这是由于1 O 2对有机阴离子中的背景阴离子具有抗性。解。然而,污染物去除的性能水平尚未得到满足,并且1 O 2生成的机理仍然难以捉摸。在此,我们首次报道了独特的硅结合杂原子(即,N)掺杂的碳(Si / N–C)纳米材料用于PMS活化,在去除各种污染物方面表现出卓越的性能,工作pH范围在2.5至11之间。例如,Si / N–C @ PMS系统实现的反应速率(0.64 min -1)远高于Si–C(0.047 min -1),NC(0.017 min -1),SiO 2(0.021 min)-1)和Co 3 O 4(0.026 min -1;标准催化剂)系统。此外,电子自旋共振(ESR)和清除实验的结果有力地证明了1 O 2的机理。代在该系统中是通过超氧自由基的O-重组2 ˙ - ,很少报道的无金属,催化剂活化的PMS系统的路径。更重要的是,我们观察到阴离子的表观抑制效果(例如,HCO 3 -,HPO 4 2-和PO 4 3-)的降解性能,具有许多以前的报告表明不一致1 Ò 2介导的氧化系统具有在这些阴离子的存在下对污染物降解的高选择性。与阴离子,含Cl进一步的研究-,HCO 3- ,和H X PO 4 (3- X)( X = 0,1,2),表明-O之间的反应2 ˙ -和这些阴离子为异常盐效应的主要原因。这项工作将加深对理解1 Ò 2形成通过中间的O- 2 ˙ -并提供新的洞察其盐抗性的能力。
更新日期:2020-10-17
中文翻译:

独特的硅掺杂的碳纳米催化剂,用于过氧单硫酸盐(PMS)活化:洞察单线态氧生成机理和异常盐效应
通过涉及单线态氧(1 O 2)的非自由基氧化途径进行的杂原子掺杂碳活化过氧单硫酸盐(PMS)系统代表了一种有前途的高级氧化过程(AOP),这是由于1 O 2对有机阴离子中的背景阴离子具有抗性。解。然而,污染物去除的性能水平尚未得到满足,并且1 O 2生成的机理仍然难以捉摸。在此,我们首次报道了独特的硅结合杂原子(即,N)掺杂的碳(Si / N–C)纳米材料用于PMS活化,在去除各种污染物方面表现出卓越的性能,工作pH范围在2.5至11之间。例如,Si / N–C @ PMS系统实现的反应速率(0.64 min -1)远高于Si–C(0.047 min -1),NC(0.017 min -1),SiO 2(0.021 min)-1)和Co 3 O 4(0.026 min -1;标准催化剂)系统。此外,电子自旋共振(ESR)和清除实验的结果有力地证明了1 O 2的机理。代在该系统中是通过超氧自由基的O-重组2 ˙ - ,很少报道的无金属,催化剂活化的PMS系统的路径。更重要的是,我们观察到阴离子的表观抑制效果(例如,HCO 3 -,HPO 4 2-和PO 4 3-)的降解性能,具有许多以前的报告表明不一致1 Ò 2介导的氧化系统具有在这些阴离子的存在下对污染物降解的高选择性。与阴离子,含Cl进一步的研究-,HCO 3- ,和H X PO 4 (3- X)( X = 0,1,2),表明-O之间的反应2 ˙ -和这些阴离子为异常盐效应的主要原因。这项工作将加深对理解1 Ò 2形成通过中间的O- 2 ˙ -并提供新的洞察其盐抗性的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号