当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction between Li, Ultrathin Adsorbed Ethylene Carbonate Films, and CoO(111) Thin Films: A Model Study of the Solid Electrolyte Interphase Formation at CoO Anodes
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.jpcc.0c06015 Jihyun Kim 1 , Florian Buchner 1 , R. Jürgen Behm 1, 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.jpcc.0c06015 Jihyun Kim 1 , Florian Buchner 1 , R. Jürgen Behm 1, 2
Affiliation
|
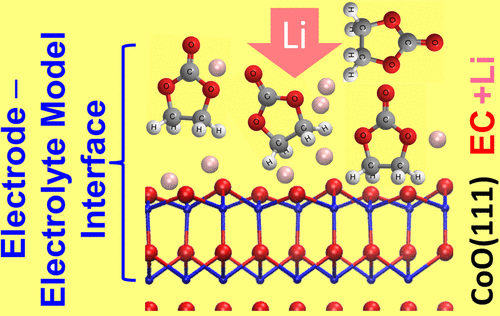
Aiming at a detailed understanding of the formation of the solid electrolyte interphase (SEI) at the electrode–electrolyte interface, which plays a critical role in the performance of Li-ion batteries (LIBs), we studied the interaction between Li, ultrathin films of ethylene carbonate (EC, main electrolyte component in LIBs), and CoO(111) thin films grown on Ru(0001), where the latter serves as a model for a conversion electrode, under ultrahigh vacuum conditions. Employing X-ray photoelectron spectroscopy, ultraviolet photoelectron spectroscopy, and Fourier transform infrared spectroscopy, we found that vapor deposition of EC on CoO(111) at 80 K results in partial decomposition of EC for films in the monolayer range, most likely because of interaction with defect sites, while it adsorbs molecularly in the multilayer regime. In both cases, desorption sets in at 170 K. Between 220 and 240 K, competing desorption and decomposition take place. To mimic the electrolyte, 0.5–2 ML of Li was stepwise postdeposited on a preadsorbed EC adlayer at 80 K and at 300 K, which leads to EC decomposition, most likely into Li-containing −C═O, −C–O–C–, −C–H, −C–C– species, and Li2O2 or LiOH. This can be considered as the initial stage of the chemical SEI formation (open-circuit conditions). CoO conversion, which is essential for Li storage in the electrode, is observed after postdeposition of Li onto a surface precovered with EC decomposition products at 300 K. In these measurements, we could resolve molecular details on the SEI formation on a CoO model anode and on the conversion of CoO, both of which are important processes in conversion-based LIBs.
中文翻译:

Li,超薄吸附的碳酸亚乙酯薄膜和CoO(111)薄膜之间的相互作用:在CoO阳极上形成固态电解质界面的模型研究
为了详细了解电极-电解质界面处的固体电解质中间相(SEI)的形成对锂离子电池(LIBs)的性能起着至关重要的作用,我们研究了Li,碳酸亚乙酯(EC,LIB中的主要电解质成分),以及在Ru(0001)上生长的CoO(111)薄膜,后者在超高真空条件下用作转换电极的模型。利用X射线光电子能谱,紫外光电子能谱和傅里叶变换红外光谱,我们发现EC在CoO(111)上80 K时气相沉积会导致EC在单层范围内部分分解,这很可能是由于相互作用带有缺陷位点,同时它在多层状态下分子吸附。在这两种情况下 解吸设定为170K。在220和240 K之间,发生竞争性解吸和分解。为了模拟电解质,将0.5–2 ML的Li在80 K和300 K的条件下逐步后沉积在预吸附的EC吸附层上,这会导致EC分解,最有可能分解成含Li的-C═O,-C-O-C –,– C–H,–CC–C–种类和Li2 O 2或LiOH。这可以被视为化学SEI形成的初始阶段(开路条件)。在将Li后沉积到预先沉积有300 K EC分解产物的表面上之后,观察到了CoO转化,这对于Li在电极中的存储至关重要。在这些测量中,我们可以解析CoO模型阳极上SEI形成的分子细节。关于CoO的转换,这两个都是基于转换的LIB的重要过程。
更新日期:2020-10-02
中文翻译:

Li,超薄吸附的碳酸亚乙酯薄膜和CoO(111)薄膜之间的相互作用:在CoO阳极上形成固态电解质界面的模型研究
为了详细了解电极-电解质界面处的固体电解质中间相(SEI)的形成对锂离子电池(LIBs)的性能起着至关重要的作用,我们研究了Li,碳酸亚乙酯(EC,LIB中的主要电解质成分),以及在Ru(0001)上生长的CoO(111)薄膜,后者在超高真空条件下用作转换电极的模型。利用X射线光电子能谱,紫外光电子能谱和傅里叶变换红外光谱,我们发现EC在CoO(111)上80 K时气相沉积会导致EC在单层范围内部分分解,这很可能是由于相互作用带有缺陷位点,同时它在多层状态下分子吸附。在这两种情况下 解吸设定为170K。在220和240 K之间,发生竞争性解吸和分解。为了模拟电解质,将0.5–2 ML的Li在80 K和300 K的条件下逐步后沉积在预吸附的EC吸附层上,这会导致EC分解,最有可能分解成含Li的-C═O,-C-O-C –,– C–H,–CC–C–种类和Li2 O 2或LiOH。这可以被视为化学SEI形成的初始阶段(开路条件)。在将Li后沉积到预先沉积有300 K EC分解产物的表面上之后,观察到了CoO转化,这对于Li在电极中的存储至关重要。在这些测量中,我们可以解析CoO模型阳极上SEI形成的分子细节。关于CoO的转换,这两个都是基于转换的LIB的重要过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号