当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioactive Alkaloids from the Actinomycete Actinoalloteichus sp. ZZ1866.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.jnatprod.0c00588 Le Qin 1 , Wenwen Yi 1 , Xiao-Yuan Lian 2 , Zhizhen Zhang 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.jnatprod.0c00588 Le Qin 1 , Wenwen Yi 1 , Xiao-Yuan Lian 2 , Zhizhen Zhang 1
Affiliation
|
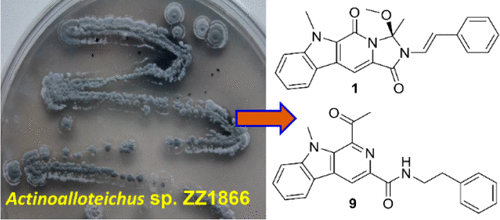
The new alkaloids marinacarbolines E–Q (1–10, 12–14), caerulomycin N (15), and actinoallonaphthyridine A (16), together with the known marinacarboline C (11) and cyanogramide (17), were isolated from the actinomycete Actinoalloteichus sp. ZZ1866. The structures of the isolated compounds were elucidated based on their HRESIMS data, extensive NMR spectroscopic analyses, Mosher’s method, ECD calculations, single-crystal X-ray diffraction analysis, and chemical degradation studies. Marinacarbolines E–L (1–8) share an indole-pyridone-imidazole tetracyclic skeleton, which is the first example of this kind of skeleton. Caerulomycin N (15) and cyanogramide (17) exhibited cytotoxic activity against both human glioma U251 and U87MG cells with IC50 values of 2.0–7.2 μM. Marinacarbolines E (1), G (3), I (5), and M (9) showed cytotoxic activity against U87MG cells with IC50 values of 2.3–8.9 μM.
中文翻译:

来自放线菌 Actinoalloteichus sp. 的生物活性生物碱。ZZ1866。
新的生物碱 marinacarbolines E–Q ( 1 – 10 , 12 – 14 )、caerulomycin N ( 15 ) 和放线基萘啶 A ( 16 ) 以及已知的 marinacarboline C ( 11 ) 和氰基胺 ( 17 ) 都从放线菌中分离出来放线菌属 ZZ1866。基于 HRESIMS 数据、广泛的 NMR 光谱分析、Mosher 方法、ECD 计算、单晶 X 射线衍射分析和化学降解研究,阐明了分离化合物的结构。Marinacarbolines E–L ( 1 – 8 ) 共享一个吲哚-吡啶酮-咪唑四环骨架,是此类骨架的首例。Caerulomycin N ( 15 ) 和 cyanogramide ( 17 ) 对人神经胶质瘤 U251 和 U87MG 细胞均表现出细胞毒活性,IC 50值为 2.0–7.2 μM。Marinacarbolines E ( 1 )、G ( 3 )、 I ( 5 ) 和 M ( 9 ) 显示出对 U87MG 细胞的细胞毒活性,IC 50值为 2.3–8.9 μM。
更新日期:2020-09-25
中文翻译:

来自放线菌 Actinoalloteichus sp. 的生物活性生物碱。ZZ1866。
新的生物碱 marinacarbolines E–Q ( 1 – 10 , 12 – 14 )、caerulomycin N ( 15 ) 和放线基萘啶 A ( 16 ) 以及已知的 marinacarboline C ( 11 ) 和氰基胺 ( 17 ) 都从放线菌中分离出来放线菌属 ZZ1866。基于 HRESIMS 数据、广泛的 NMR 光谱分析、Mosher 方法、ECD 计算、单晶 X 射线衍射分析和化学降解研究,阐明了分离化合物的结构。Marinacarbolines E–L ( 1 – 8 ) 共享一个吲哚-吡啶酮-咪唑四环骨架,是此类骨架的首例。Caerulomycin N ( 15 ) 和 cyanogramide ( 17 ) 对人神经胶质瘤 U251 和 U87MG 细胞均表现出细胞毒活性,IC 50值为 2.0–7.2 μM。Marinacarbolines E ( 1 )、G ( 3 )、 I ( 5 ) 和 M ( 9 ) 显示出对 U87MG 细胞的细胞毒活性,IC 50值为 2.3–8.9 μM。











































 京公网安备 11010802027423号
京公网安备 11010802027423号