当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental and Predictive Equilibrium Thermodynamics of the Aqueous Ternary System (LiCl + CaCl2 + H2O) at T = 288.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.jced.0c00118 Long Li 1 , Fei Yuan 1 , Yafei Guo 1 , Sisi Zhang 1 , Tianlong Deng 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-08-31 , DOI: 10.1021/acs.jced.0c00118 Long Li 1 , Fei Yuan 1 , Yafei Guo 1 , Sisi Zhang 1 , Tianlong Deng 1
Affiliation
|
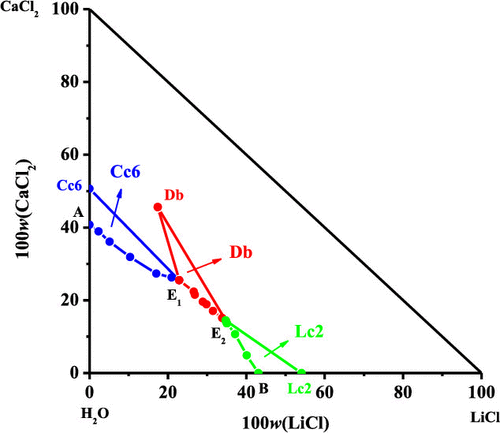
As an energy metal, lithium is widely used in many fields. With the depletion of the solid mineral resource, to extract and recover lithium from liquid brines is going toward an inevitable trend in the future. In order to utilize the worthy lithium-containing brine resources in the world efficiently, the experimental equilibrium solubility and the solution physicochemical properties including density (ρ), refractive index (nD), and pH for the aqueous ternary system (LiCl + CaCl2 + H2O) at T = 288.15 K were determined using the isothermal dissolution equilibrium method. In addition, the calculated values of refractive index and density obtained by using empirical equations agree well with the experimental data. According to the Pitzer extended Harvie and Weare model (HW model) of electrolyte ion-interaction theory, the single-salt parameters β(0), β(1), and Cϕ of LiCl and CaCl2, the mixing ion-interaction parameters θLi,Ca and ΨLi,Ca,Cl, and the equilibrium constants of solid phases LiCl·2H2O, CaCl2·6H2O, and LiCl·CaCl2·5H2O existed in the system at 288.15 K were obtained for the first time. The theoretical predictive equilibrium solubilities for this ternary system at 288.15 K agree well with the experimental value, and this result indicates that the Pitzer single-salt parameters, the mixing ion-interaction parameters, and the equilibrium constants obtained in this work are reliable. The results for the system containing lithium in this study are essential for the development of universal thermodynamic models for brine systems containing lithium.
中文翻译:

三元体系(LiCl + CaCl 2 + H 2 O)在T = 288.15 K时的实验和预测平衡热力学
作为一种能源金属,锂被广泛应用于许多领域。随着固体矿物质资源的枯竭,从液态盐水中提取和回收锂的趋势正在走向未来。为了有效地利用世界上有价值的含锂盐水资源,三元水溶液体系(LiCl + CaCl 2)的实验平衡溶解度和溶液的理化性质包括密度(ρ),折射率(n D)和pH。+ H 2 O)在T使用等温溶解平衡方法确定= 288.15K。另外,通过经验公式获得的折射率和密度的计算值与实验数据很好地吻合。根据皮特泽延伸哈维和电解质离子相互作用理论的Weare模型(HW模型),单盐参数β (0),β (1) ,和Ç φ的LiCl和CaCl的2,混合离子相互作用参数θLi ,Ca和ΨLi ,Ca,Cl以及固相LiCl·2H 2 O,CaCl 2 ·6H 2 O和LiCl·CaCl 2 ·5H 2的平衡常数首次获得288.15 K时系统中存在的O。该三元体系在288.15 K时的理论预测平衡溶解度与实验值非常吻合,该结果表明在这项工作中获得的Pitzer单盐参数,混合离子相互作用参数以及平衡常数是可靠的。本研究中含锂系统的结果对于开发含锂盐水系统的通用热力学模型至关重要。
更新日期:2020-09-10
中文翻译:

三元体系(LiCl + CaCl 2 + H 2 O)在T = 288.15 K时的实验和预测平衡热力学
作为一种能源金属,锂被广泛应用于许多领域。随着固体矿物质资源的枯竭,从液态盐水中提取和回收锂的趋势正在走向未来。为了有效地利用世界上有价值的含锂盐水资源,三元水溶液体系(LiCl + CaCl 2)的实验平衡溶解度和溶液的理化性质包括密度(ρ),折射率(n D)和pH。+ H 2 O)在T使用等温溶解平衡方法确定= 288.15K。另外,通过经验公式获得的折射率和密度的计算值与实验数据很好地吻合。根据皮特泽延伸哈维和电解质离子相互作用理论的Weare模型(HW模型),单盐参数β (0),β (1) ,和Ç φ的LiCl和CaCl的2,混合离子相互作用参数θLi ,Ca和ΨLi ,Ca,Cl以及固相LiCl·2H 2 O,CaCl 2 ·6H 2 O和LiCl·CaCl 2 ·5H 2的平衡常数首次获得288.15 K时系统中存在的O。该三元体系在288.15 K时的理论预测平衡溶解度与实验值非常吻合,该结果表明在这项工作中获得的Pitzer单盐参数,混合离子相互作用参数以及平衡常数是可靠的。本研究中含锂系统的结果对于开发含锂盐水系统的通用热力学模型至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号