当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen-Free Regioselective Biocatalytic Demethylation of Methyl-phenyl Ethers via Methyltransfer Employing Veratrol-O-demethylase
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-08-31 , DOI: 10.1021/acscatal.0c02790 Christopher Grimm 1 , Mattia Lazzarotto 1 , Simona Pompei 1 , Johanna Schichler 1 , Nina Richter 2 , Judith E Farnberger 1, 2 , Michael Fuchs 1 , Wolfgang Kroutil 1, 3, 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-08-31 , DOI: 10.1021/acscatal.0c02790 Christopher Grimm 1 , Mattia Lazzarotto 1 , Simona Pompei 1 , Johanna Schichler 1 , Nina Richter 2 , Judith E Farnberger 1, 2 , Michael Fuchs 1 , Wolfgang Kroutil 1, 3, 4
Affiliation
|
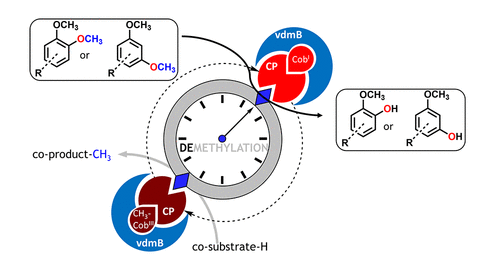
The cleavage of aryl methyl ethers is a common reaction in chemistry requiring rather harsh conditions; consequently, it is prone to undesired reactions and lacks regioselectivity. Nevertheless, O-demethylation of aryl methyl ethers is a tool to valorize natural and pharmaceutical compounds by deprotecting reactive hydroxyl moieties. Various oxidative enzymes are known to catalyze this reaction at the expense of molecular oxygen, which may lead in the case of phenols/catechols to undesired side reactions (e.g., oxidation, polymerization). Here an oxygen-independent demethylation via methyl transfer is presented employing a cobalamin-dependent veratrol-O-demethylase (vdmB). The biocatalytic demethylation transforms a variety of aryl methyl ethers with two functional methoxy moieties either in 1,2-position or in 1,3-position. Biocatalytic reactions enabled, for instance, the regioselective monodemethylation of substituted 3,4-dimethoxy phenol as well as the monodemethylation of 1,3,5-trimethoxybenzene. The methyltransferase vdmB was also successfully applied for the regioselective demethylation of natural compounds such as papaverine and rac-yatein. The approach presented here represents an alternative to chemical and enzymatic demethylation concepts and allows performing regioselective demethylation in the absence of oxygen under mild conditions, representing a valuable extension of the synthetic repertoire to modify pharmaceuticals and diversify natural products.
中文翻译:

使用藜芦醇-O-去甲基酶通过甲基转移对甲基-苯基醚进行无氧区域选择性生物催化去甲基化
芳基甲基醚的裂解是化学中的常见反应,需要相当苛刻的条件;因此,它容易发生不需要的反应并且缺乏区域选择性。然而,芳基甲基醚的O-去甲基化是一种通过去保护活性羟基部分来使天然和药物化合物增值的工具。已知各种氧化酶以分子氧为代价催化该反应,这在酚类/儿茶酚类的情况下可能导致不希望的副反应(例如氧化、聚合)。此处介绍了通过甲基转移进行的非氧依赖性去甲基化,采用钴胺素依赖性藜芦醇-O-脱甲基酶 (vdmB)。生物催化去甲基化转化了多种芳基甲基醚,它们在 1,2-位或 1,3-位具有两个功能性甲氧基部分。例如,生物催化反应使取代的 3,4-二甲氧基苯酚的区域选择性单去甲基化以及 1,3,5-三甲氧基苯的单去甲基化成为可能。甲基转移酶 vdmB 也成功应用于罂粟碱和外消旋等天然化合物的区域选择性去甲基化。-雅廷。这里介绍的方法代表了化学和酶促去甲基化概念的替代方案,并允许在温和条件下在无氧的情况下进行区域选择性去甲基化,代表了合成库的宝贵扩展,以修饰药物和使天然产物多样化。
更新日期:2020-09-20
中文翻译:

使用藜芦醇-O-去甲基酶通过甲基转移对甲基-苯基醚进行无氧区域选择性生物催化去甲基化
芳基甲基醚的裂解是化学中的常见反应,需要相当苛刻的条件;因此,它容易发生不需要的反应并且缺乏区域选择性。然而,芳基甲基醚的O-去甲基化是一种通过去保护活性羟基部分来使天然和药物化合物增值的工具。已知各种氧化酶以分子氧为代价催化该反应,这在酚类/儿茶酚类的情况下可能导致不希望的副反应(例如氧化、聚合)。此处介绍了通过甲基转移进行的非氧依赖性去甲基化,采用钴胺素依赖性藜芦醇-O-脱甲基酶 (vdmB)。生物催化去甲基化转化了多种芳基甲基醚,它们在 1,2-位或 1,3-位具有两个功能性甲氧基部分。例如,生物催化反应使取代的 3,4-二甲氧基苯酚的区域选择性单去甲基化以及 1,3,5-三甲氧基苯的单去甲基化成为可能。甲基转移酶 vdmB 也成功应用于罂粟碱和外消旋等天然化合物的区域选择性去甲基化。-雅廷。这里介绍的方法代表了化学和酶促去甲基化概念的替代方案,并允许在温和条件下在无氧的情况下进行区域选择性去甲基化,代表了合成库的宝贵扩展,以修饰药物和使天然产物多样化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号