当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The structure of myeloid cell‐specific TNF inhibitors affects their biological properties
FEBS Letters ( IF 3.0 ) Pub Date : 2020-09-04 , DOI: 10.1002/1873-3468.13913 Ekaterina A Vasilenko 1 , Ekaterina N Gorshkova 1 , Irina V Astrakhantseva 1, 2 , Marina S Drutskaya 3 , Sergei V Tillib 4 , Sergei A Nedospasov 2, 3, 5 , Vladislav V Mokhonov 3, 6
FEBS Letters ( IF 3.0 ) Pub Date : 2020-09-04 , DOI: 10.1002/1873-3468.13913 Ekaterina A Vasilenko 1 , Ekaterina N Gorshkova 1 , Irina V Astrakhantseva 1, 2 , Marina S Drutskaya 3 , Sergei V Tillib 4 , Sergei A Nedospasov 2, 3, 5 , Vladislav V Mokhonov 3, 6
Affiliation
|
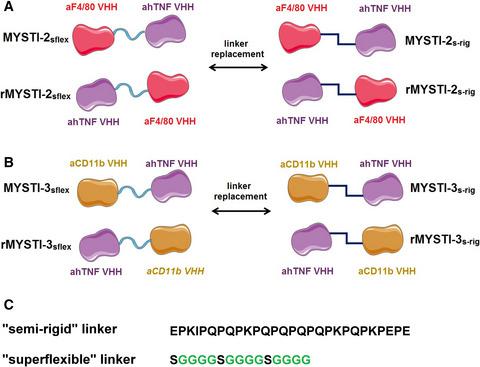
Spatial organization and conformational changes of antibodies may significantly affect their biological functions. We assessed the effect of mutual organization of the two VHH domains within bispecific antibodies recognizing human TNF and the surface molecules of murine myeloid cells (F4/80 or CD11b) on TNF retention and inhibition. TNF‐neutralizing properties in vitro and in vivo of MYSTI‐2 and MYSTI‐3 antibodies were compared with new variants with interchanged VHH domains and different linker sequences. The most effective structure of MYSTI‐2 and MYSTI‐3 proteins required the Ser/Gly‐containing ‘superflexible’ linker. The orientation of the modules was crucial for the activity of the proteins, but not for MYSTI‐3 with the Pro/Gln‐containing ‘semi‐rigid’ linker. Our results may contribute toward the development of more effective drug prototypes.
中文翻译:

骨髓细胞特异性 TNF 抑制剂的结构影响其生物学特性
抗体的空间组织和构象变化可能会显着影响其生物学功能。我们评估了识别人 TNF 和鼠骨髓细胞(F4/80 或 CD11b)的表面分子的双特异性抗体内两个 VHH 结构域的相互组织对 TNF 保留和抑制的影响。将 MYSTI-2 和 MYSTI-3 抗体的体外和体内 TNF 中和特性与具有互换 VHH 结构域和不同接头序列的新变体进行比较。MYSTI-2 和 MYSTI-3 蛋白的最有效结构需要含有 Ser/Gly 的“超柔性”接头。模块的方向对蛋白质的活性至关重要,但对于带有 Pro/Gln 的“半刚性”接头的 MYSTI-3 则不然。我们的结果可能有助于开发更有效的药物原型。
更新日期:2020-09-04
中文翻译:

骨髓细胞特异性 TNF 抑制剂的结构影响其生物学特性
抗体的空间组织和构象变化可能会显着影响其生物学功能。我们评估了识别人 TNF 和鼠骨髓细胞(F4/80 或 CD11b)的表面分子的双特异性抗体内两个 VHH 结构域的相互组织对 TNF 保留和抑制的影响。将 MYSTI-2 和 MYSTI-3 抗体的体外和体内 TNF 中和特性与具有互换 VHH 结构域和不同接头序列的新变体进行比较。MYSTI-2 和 MYSTI-3 蛋白的最有效结构需要含有 Ser/Gly 的“超柔性”接头。模块的方向对蛋白质的活性至关重要,但对于带有 Pro/Gln 的“半刚性”接头的 MYSTI-3 则不然。我们的结果可能有助于开发更有效的药物原型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号