当前位置:
X-MOL 学术
›
J. Polym. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
De‐tert‐butylation of poly(N‐tert‐butyl‐N‐n‐propylacrylamide): Stereochemical analysis at the triad level
Journal of Polymer Science ( IF 3.9 ) Pub Date : 2020-08-31 , DOI: 10.1002/pol.20200473 Tomohiro Hirano 1 , Misato Sugiura 1 , Ryuya Endo 1 , Miyuki Oshimura 1 , Koichi Ute 1
Journal of Polymer Science ( IF 3.9 ) Pub Date : 2020-08-31 , DOI: 10.1002/pol.20200473 Tomohiro Hirano 1 , Misato Sugiura 1 , Ryuya Endo 1 , Miyuki Oshimura 1 , Koichi Ute 1
Affiliation
|
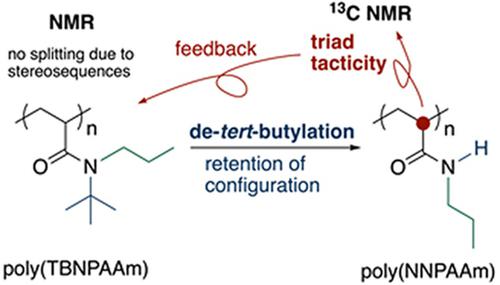
The stereochemical analysis of polymers derived from N,N‐disubstituted acrylamides is usually difficult. The diad tacticity can be determined from the 1H nuclear magnetic resonance (NMR) signals of the main‐chain methylene groups. However, the splitting because of the configurational sequences is poor, even in 13C NMR, which does not allow determination of the tacticity at the triad level. In contrast, the stereochemical analysis of polymers derived from N‐monosubstituted acrylamides is easily conducted and the triad tacticity can be determined from the 13C signals of the main‐chain methine groups. Thus, stereochemical analysis of N,N‐disubstituted polymers should be able to be conducted if the polymers are transformed into N‐monosubstituted polymers with retention of the configurational sequence. Poly(N‐tert‐butyl‐N‐n‐propylacrylamide) was radically prepared, and de‐tert‐butylation was conducted by treatment with scandium triflate in a mixed solvent of CH3CN and 1,4‐dioxane at 50, 80, and 110°C. 1H NMR analysis of the resulting polymers indicated quantitative conversion after 72 hr, regardless of the temperature. 13C NMR analysis of the transformed polymers confirmed that the configurational sequences were retained during the reaction. Thus, the triad stereochemical analysis of N,N‐disubstituted polymers was successfully conducted by de‐tert‐butylation as a polymer reaction, followed by 13C NMR analysis of the transformed polymers.
中文翻译:

聚(N-叔丁基-N-正丙基丙烯酰胺)的叔叔丁基化:三重态水平的立体化学分析
通常很难对衍生自N,N-二取代丙烯酰胺的聚合物进行立体化学分析。双立构规整度可以通过主链亚甲基的1 H核磁共振(NMR)信号确定。然而,即使在13 C NMR中,由于构型序列的分裂也很差,这不能确定三单元组水平的立构规整度。相反,从N-单取代的丙烯酰胺衍生的聚合物的立体化学分析很容易进行,而三单元组立构规整度可以从主链次甲基的13 C信号确定。因此,对N,N的立体化学分析如果将聚合物转变为具有构象序列的N-单取代聚合物,则应能够进行-di取代的聚合物。聚(Ñ -叔丁基- ñ - ñ -propylacrylamide)中的溶液自由基制备,并且去叔通过用三氟甲磺酸钪治疗CH的混合溶剂中进行-butylation 3 CN,并在50 1,4-二恶烷,80%,和110°C。所得聚合物的1 H NMR分析表明,无论温度如何,在72小时后都发生了定量转化。13转化的聚合物的13 C NMR分析证实在反应过程中保留了构型序列。因此,通过将叔丁基化反应作为聚合物反应成功地进行了N,N-二取代聚合物的三单元组立体化学分析,然后对转化的聚合物进行了13 C NMR分析。
更新日期:2020-10-17
中文翻译:

聚(N-叔丁基-N-正丙基丙烯酰胺)的叔叔丁基化:三重态水平的立体化学分析
通常很难对衍生自N,N-二取代丙烯酰胺的聚合物进行立体化学分析。双立构规整度可以通过主链亚甲基的1 H核磁共振(NMR)信号确定。然而,即使在13 C NMR中,由于构型序列的分裂也很差,这不能确定三单元组水平的立构规整度。相反,从N-单取代的丙烯酰胺衍生的聚合物的立体化学分析很容易进行,而三单元组立构规整度可以从主链次甲基的13 C信号确定。因此,对N,N的立体化学分析如果将聚合物转变为具有构象序列的N-单取代聚合物,则应能够进行-di取代的聚合物。聚(Ñ -叔丁基- ñ - ñ -propylacrylamide)中的溶液自由基制备,并且去叔通过用三氟甲磺酸钪治疗CH的混合溶剂中进行-butylation 3 CN,并在50 1,4-二恶烷,80%,和110°C。所得聚合物的1 H NMR分析表明,无论温度如何,在72小时后都发生了定量转化。13转化的聚合物的13 C NMR分析证实在反应过程中保留了构型序列。因此,通过将叔丁基化反应作为聚合物反应成功地进行了N,N-二取代聚合物的三单元组立体化学分析,然后对转化的聚合物进行了13 C NMR分析。











































 京公网安备 11010802027423号
京公网安备 11010802027423号