当前位置:
X-MOL 学术
›
Mater. Corros.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The kinetics of copper corrosion in nitric acid
Materials and Corrosion ( IF 1.6 ) Pub Date : 2020-08-31 , DOI: 10.1002/maco.202011707 Joseph Turnbull 1 , Ryan Szukalo 1 , Dmitrij Zagidulin 1 , Mark Biesinger 2 , David Shoesmith 1, 2
Materials and Corrosion ( IF 1.6 ) Pub Date : 2020-08-31 , DOI: 10.1002/maco.202011707 Joseph Turnbull 1 , Ryan Szukalo 1 , Dmitrij Zagidulin 1 , Mark Biesinger 2 , David Shoesmith 1, 2
Affiliation
|
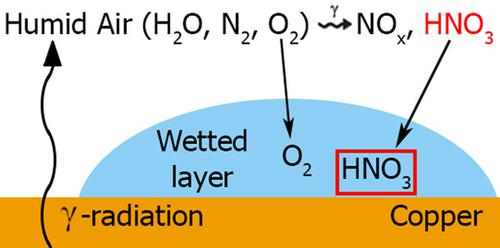
The strategy for the permanent disposal of high‐level nuclear waste in Canada involves sealing it in a copper‐coated steel container and burying it in a deep geologic repository. During the early emplacement period, the container could be exposed to warm humid air, which could result in the condensation of nitric acid, produced by the radiolysis of the humid air, on the copper surface. Previous studies have suggested that both nitrate and oxygen reduction will drive copper corrosion, with the nitrate reduction kinetics being dependent on the concentration of soluble copper(I) produced by the anodic dissolution of copper in the reaction with oxygen. This study focused on determining the kinetics of nitrate and oxygen reduction and elucidating the synergistic relationship between the two processes. This was investigated using corrosion potential and polarization measurements in conjunction with scanning electron microscopy and X‐ray photoelectron spectroscopy. Oxygen reduction was shown to be the dominant cathodic reaction with the oxidation of copper(I) to copper(II) by nitrate, promoting the catalytic cycle involving the reaction of copper(II) with copper to reproduce copper(I).
中文翻译:

硝酸中铜腐蚀的动力学
在加拿大,永久性处置高级别核废料的策略包括将其密封在镀铜钢容器中,然后将其掩埋在深层的地质处置库中。在放置初期,容器可能会暴露在温暖的潮湿空气中,这可能导致硝酸盐的凝结,而硝酸是由潮湿空气的辐射分解在铜表面上凝结的。先前的研究表明,硝酸盐和氧气的还原都会推动铜的腐蚀,硝酸盐的还原动力学取决于与氧反应中铜的阳极溶解所产生的可溶性铜(I)的浓度。这项研究的重点是确定硝酸盐和氧气还原的动力学,并阐明这两个过程之间的协同关系。使用腐蚀电势和极化测量以及扫描电子显微镜和X射线光电子能谱对此进行了研究。氧还原反应是硝酸盐将铜(I)氧化为铜(II)的主要阴极反应,从而促进了催化循环,涉及铜(II)与铜反应生成铜(I)。
更新日期:2020-08-31
中文翻译:

硝酸中铜腐蚀的动力学
在加拿大,永久性处置高级别核废料的策略包括将其密封在镀铜钢容器中,然后将其掩埋在深层的地质处置库中。在放置初期,容器可能会暴露在温暖的潮湿空气中,这可能导致硝酸盐的凝结,而硝酸是由潮湿空气的辐射分解在铜表面上凝结的。先前的研究表明,硝酸盐和氧气的还原都会推动铜的腐蚀,硝酸盐的还原动力学取决于与氧反应中铜的阳极溶解所产生的可溶性铜(I)的浓度。这项研究的重点是确定硝酸盐和氧气还原的动力学,并阐明这两个过程之间的协同关系。使用腐蚀电势和极化测量以及扫描电子显微镜和X射线光电子能谱对此进行了研究。氧还原反应是硝酸盐将铜(I)氧化为铜(II)的主要阴极反应,从而促进了催化循环,涉及铜(II)与铜反应生成铜(I)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号