当前位置:
X-MOL 学术
›
Genes Cells
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cdk1 negatively regulates the spindle localization of Prc1 in mouse oocytes.
Genes to Cells ( IF 1.3 ) Pub Date : 2020-08-31 , DOI: 10.1111/gtc.12803 Sui Nishiyama 1, 2 , Shuhei Yoshida 1 , Tomoya S Kitajima 1, 2
Genes to Cells ( IF 1.3 ) Pub Date : 2020-08-31 , DOI: 10.1111/gtc.12803 Sui Nishiyama 1, 2 , Shuhei Yoshida 1 , Tomoya S Kitajima 1, 2
Affiliation
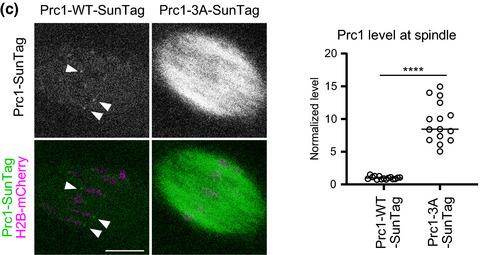
|
Chromosome segregation requires the formation of a bipolar spindle. The timely bipolarization of the acentrosomal spindle during meiosis I in mouse oocytes depends on the antiparallel microtubule crosslinker Prc1. How Prc1 is regulated in oocytes remains poorly understood. In this study, we show that the kinase Cdk1 negatively regulates the spindle localization of Prc1 in mouse oocytes. The acute inhibition of Cdk1 activity led to excessive localization of Prc1 at the spindle and kinetochores, whereas the overactivation of Cdk1 had opposite effects. The overexpression of Prc1 carrying mutations at Cdk1‐mediated phosphorylation sites increased its localization to the spindle, accelerated spindle bipolarization and caused spindle‐checkpoint‐dependent arrest at metaphase I. Overactivation of Cdk1 delayed spindle bipolarization, which was reversed by the overexpression of a phospho‐mutant form but not the wild‐type form of Prc1. These results suggest that Cdk1‐mediated phosphorylation negatively regulates Prc1 localization to ensure the timely bipolarization of the acentrosomal spindle during meiosis I in mammalian oocytes.
中文翻译:

Cdk1负调节小鼠卵母细胞中Prc1的纺锤体定位。
染色体分离需要形成双极纺锤体。在小鼠卵母细胞减数分裂I期间,人质体纺锤体的及时双极化取决于反平行微管交联剂Prc1。卵母细胞中如何调节Prc1仍然知之甚少。在这项研究中,我们表明激酶Cdk1负调节小鼠卵母细胞中Prc1的纺锤体定位。Cdk1活性的急性抑制导致Prc1在纺锤体和动植物体内的过度定位,而Cdk1的过度激活具有相反的作用。在Cdk1介导的磷酸化位点上携带突变的Prc1的过表达增加了其在纺锤体中的定位,加速了纺锤体双极化,并导致了中期I的纺锤体检查点依赖性停滞。Cdk1的过度激活会延迟纺锤体双极化,Prc1的磷酸化突变体形式的过表达而野生型形式则没有逆转。这些结果表明,Cdk1介导的磷酸化负调控Prc1的定位,以确保在哺乳动物卵母细胞减数分裂I期间及时地对人孔纺锤体进行双极化。
更新日期:2020-10-19
中文翻译:

Cdk1负调节小鼠卵母细胞中Prc1的纺锤体定位。
染色体分离需要形成双极纺锤体。在小鼠卵母细胞减数分裂I期间,人质体纺锤体的及时双极化取决于反平行微管交联剂Prc1。卵母细胞中如何调节Prc1仍然知之甚少。在这项研究中,我们表明激酶Cdk1负调节小鼠卵母细胞中Prc1的纺锤体定位。Cdk1活性的急性抑制导致Prc1在纺锤体和动植物体内的过度定位,而Cdk1的过度激活具有相反的作用。在Cdk1介导的磷酸化位点上携带突变的Prc1的过表达增加了其在纺锤体中的定位,加速了纺锤体双极化,并导致了中期I的纺锤体检查点依赖性停滞。Cdk1的过度激活会延迟纺锤体双极化,Prc1的磷酸化突变体形式的过表达而野生型形式则没有逆转。这些结果表明,Cdk1介导的磷酸化负调控Prc1的定位,以确保在哺乳动物卵母细胞减数分裂I期间及时地对人孔纺锤体进行双极化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号