Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Murine induced pluripotent stem cell-derived neuroimmune cell culture models emphasize opposite immune-effector functions of interleukin 13-primed microglia and macrophages in terms of neuroimmune toxicity.
Glia ( IF 5.4 ) Pub Date : 2020-08-31 , DOI: 10.1002/glia.23899 Alessandra Quarta 1, 2 , Tim Meese 3 , Zoë Pieters 2, 4, 5 , Elise Van Breedam 1, 2 , Debbie Le Blon 1, 2 , Jana Van Broeckhoven 6 , Sven Hendrix 6 , Herman Goossens 2 , Niel Hens 2, 4, 5 , Zwi Berneman 1, 2 , Filip Van Nieuwerburgh 3 , Peter Ponsaerts 1, 2
Glia ( IF 5.4 ) Pub Date : 2020-08-31 , DOI: 10.1002/glia.23899 Alessandra Quarta 1, 2 , Tim Meese 3 , Zoë Pieters 2, 4, 5 , Elise Van Breedam 1, 2 , Debbie Le Blon 1, 2 , Jana Van Broeckhoven 6 , Sven Hendrix 6 , Herman Goossens 2 , Niel Hens 2, 4, 5 , Zwi Berneman 1, 2 , Filip Van Nieuwerburgh 3 , Peter Ponsaerts 1, 2
Affiliation
|
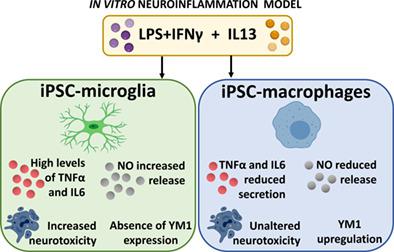
Cellular models of induced pluripotent stem cell (iPSC)‐derived microglia and macrophages are an emerging toolbox to investigate neuroinflammation in vitro. We previously demonstrated that murine iPSC‐microglia and iPSC‐macrophages display phenotypical activation properties highly comparable to microglia and macrophages in vivo. Here we extended the characterization of iPSC‐microglia and iPSC‐macrophages with the analysis of their transcriptome profile. Next, these cellular models were employed to evaluate neuroimmune toxicity in vitro and to investigate the immune‐modulatory properties of interleukin 13 (IL13), a cytokine known for its ability to protect against neuroinflammation‐induced pathology by modulating microglia and macrophage activation. iPSC‐microglia and iPSC‐macrophages, in co‐culture with astrocyte‐committed neural stem cells (NSC), were (pre)treated with IL13 and stimulated with lipopolysaccharide (LPS) and interferon γ (IFNγ), to assess how IL13 modulates their inflammatory response. Additionally, the use of luciferase‐expressing NSC (Luc‐NSC) allowed real‐time monitoring of immune‐mediated neurotoxicity. Despite the known anti‐inflammatory properties of IL13, iPSC‐microglia primed with IL13 before LPS + IFNγ stimulation significantly increased NO secretion. This was associated with a marked reduction of the luminescence signal produced by Luc‐NSC. Interestingly, we observed that IL13 signaling has a divergent functional outcome in microglia as compared to macrophages, as for the latter no major alterations in NO release and Luc‐NSC viability were observed upon IL13 (pre)treatment. Finally, the striking IL13‐induced upregulation of NO secretion by microglia under pro‐inflammatory conditions was confirmed in vivo, where intracerebral delivery of IL13 increased inducible nitric oxide synthase mRNA expression. Concluding, we applied iPSC‐derived neuroimmune cell culture models to identify distinct neuroimmune (toxicity) responses of microglia and macrophages to IL13‐based immune modulation.
中文翻译:

小鼠诱导的多能干细胞衍生的神经免疫细胞培养模型强调白细胞介素 13 引发的小胶质细胞和巨噬细胞在神经免疫毒性方面相反的免疫效应功能。
诱导多能干细胞 (iPSC) 衍生的小胶质细胞和巨噬细胞的细胞模型是体外研究神经炎症的新兴工具箱。我们之前证明,小鼠 iPSC-小胶质细胞和 iPSC-巨噬细胞在体内表现出与小胶质细胞和巨噬细胞高度可比的表型激活特性。在这里,我们通过分析它们的转录组谱扩展了 iPSC-小胶质细胞和 iPSC-巨噬细胞的表征。接下来,这些细胞模型被用于评估体外神经免疫毒性,并研究白细胞介素 13 (IL13) 的免疫调节特性,白细胞介素 13 是一种细胞因子,以通过调节小胶质细胞和巨噬细胞活化来防止神经炎症诱导的病理变化而闻名。iPSC-小胶质细胞和 iPSC-巨噬细胞与星形胶质细胞定向神经干细胞 (NSC) 共培养,用 IL13 (预) 处理并用脂多糖 (LPS) 和干扰素 γ (IFNγ) 刺激,以评估 IL13 如何调节它们的炎症反应。此外,使用表达荧光素酶的 NSC (Luc-NSC) 可以实时监测免疫介导的神经毒性。尽管 IL13 具有已知的抗炎特性,但在 LPS + IFNγ 刺激之前用 IL13 引发的 iPSC 小胶质细胞显着增加了 NO 的分泌。这与 Luc-NSC 产生的发光信号显着减少有关。有趣的是,我们观察到与巨噬细胞相比,IL13 信号传导在小胶质细胞中具有不同的功能结果,因为在 IL13(预)处理后,没有观察到 NO 释放和 Luc-NSC 活力的重大变化。最后,在体内证实了在促炎条件下,IL13 诱导的小胶质细胞对 NO 分泌的显着上调,其中 IL13 的脑内递送增加了诱导型一氧化氮合酶 mRNA 的表达。最后,
更新日期:2020-08-31
中文翻译:

小鼠诱导的多能干细胞衍生的神经免疫细胞培养模型强调白细胞介素 13 引发的小胶质细胞和巨噬细胞在神经免疫毒性方面相反的免疫效应功能。
诱导多能干细胞 (iPSC) 衍生的小胶质细胞和巨噬细胞的细胞模型是体外研究神经炎症的新兴工具箱。我们之前证明,小鼠 iPSC-小胶质细胞和 iPSC-巨噬细胞在体内表现出与小胶质细胞和巨噬细胞高度可比的表型激活特性。在这里,我们通过分析它们的转录组谱扩展了 iPSC-小胶质细胞和 iPSC-巨噬细胞的表征。接下来,这些细胞模型被用于评估体外神经免疫毒性,并研究白细胞介素 13 (IL13) 的免疫调节特性,白细胞介素 13 是一种细胞因子,以通过调节小胶质细胞和巨噬细胞活化来防止神经炎症诱导的病理变化而闻名。iPSC-小胶质细胞和 iPSC-巨噬细胞与星形胶质细胞定向神经干细胞 (NSC) 共培养,用 IL13 (预) 处理并用脂多糖 (LPS) 和干扰素 γ (IFNγ) 刺激,以评估 IL13 如何调节它们的炎症反应。此外,使用表达荧光素酶的 NSC (Luc-NSC) 可以实时监测免疫介导的神经毒性。尽管 IL13 具有已知的抗炎特性,但在 LPS + IFNγ 刺激之前用 IL13 引发的 iPSC 小胶质细胞显着增加了 NO 的分泌。这与 Luc-NSC 产生的发光信号显着减少有关。有趣的是,我们观察到与巨噬细胞相比,IL13 信号传导在小胶质细胞中具有不同的功能结果,因为在 IL13(预)处理后,没有观察到 NO 释放和 Luc-NSC 活力的重大变化。最后,在体内证实了在促炎条件下,IL13 诱导的小胶质细胞对 NO 分泌的显着上调,其中 IL13 的脑内递送增加了诱导型一氧化氮合酶 mRNA 的表达。最后,











































 京公网安备 11010802027423号
京公网安备 11010802027423号