当前位置:
X-MOL 学术
›
Microbiologyopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In vitro reconstitution and characterization of pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase hybrid complex from Corynebacterium glutamicum.
MicrobiologyOpen ( IF 3.9 ) Pub Date : 2020-08-30 , DOI: 10.1002/mbo3.1113 Hirokazu Kinugawa 1 , Naoko Kondo 1 , Ayano Komine-Abe 1 , Takeo Tomita 1, 2 , Makoto Nishiyama 1, 2 , Saori Kosono 1, 2, 3
MicrobiologyOpen ( IF 3.9 ) Pub Date : 2020-08-30 , DOI: 10.1002/mbo3.1113 Hirokazu Kinugawa 1 , Naoko Kondo 1 , Ayano Komine-Abe 1 , Takeo Tomita 1, 2 , Makoto Nishiyama 1, 2 , Saori Kosono 1, 2, 3
Affiliation
|
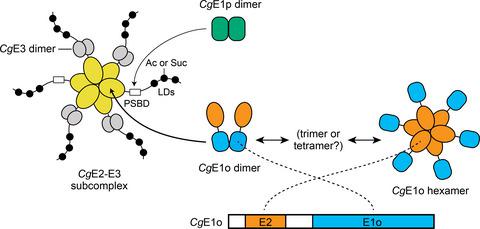
Pyruvate dehydrogenase (PDH) and 2‐oxoglutarate dehydrogenase (ODH) are critical enzymes in central carbon metabolism. In Corynebacterium glutamicum, an unusual hybrid complex consisting of CgE1p (thiamine diphosphate‐dependent pyruvate dehydrogenase, AceE), CgE2 (dihydrolipoamide acetyltransferase, AceF), CgE3 (dihydrolipoamide dehydrogenase, Lpd), and CgE1o (thiamine diphosphate‐dependent 2‐oxoglutarate dehydrogenase, OdhA) has been suggested. Here, we elucidated that the PDH‐ODH hybrid complex in C. glutamicum probably consists of six copies of CgE2 in its core, which is rather compact compared with PDH and ODH in other microorganisms that have twenty‐four copies of E2. We found that CgE2 formed a stable complex with CgE3 (CgE2‐E3 subcomplex) in vitro, hypothetically comprised of two CgE2 trimers and four CgE3 dimers. We also found that CgE1o exists mainly as a hexamer in solution and is ready to form an active ODH complex when mixed with the CgE2‐E3 subcomplex. Our in vitro reconstituted system showed CgE1p‐ and CgE1o‐dependent inhibition of ODH and PDH, respectively, actively supporting the formation of the hybrid complex, in which both CgE1p and CgE1o associate with a single CgE2‐E3. In gel filtration chromatography, all the subunits of CgODH were eluted in the same fraction, whereas CgE1p was eluted separately from CgE2‐E3, suggesting a weak association of CgE1p with CgE2 compared with that of CgE1o. This study revealed the unique molecular architecture of the hybrid complex from C. glutamicum and the compact‐sized complex would provide an advantage to determine the whole structure of the unusual hybrid complex.
中文翻译:

谷氨酸棒杆菌丙酮酸脱氢酶和2-氧戊二酸脱氢酶杂合体的体外重组和鉴定。
丙酮酸脱氢酶(PDH)和2-氧戊二酸脱氢酶(ODH)是中央碳代谢中的关键酶。在谷氨酸棒杆菌中,一种不寻常的杂合复合物,由Cg E1p(硫胺二磷酸依赖性丙酮酸脱氢酶,AceE),Cg E2(二氢脂酰胺乙酰转移酶,AceF),Cg E3(二氢脂酰胺脱氢酶,Lpd)和Cg E1o(硫胺素二磷酸依赖性2有人建议使用-oxoglutarate脱氢酶(OdhA)。在这里,我们阐明了C中的PDH-ODH杂合体。 谷氨酸可能由六份Cg组成E2的核心,与其他具有24个E2拷贝的微生物中的PDH和ODH相比,它非常紧凑。我们发现Cg E2在体外与Cg E3形成了稳定的复合物(Cg E2-E3亚复合物),假设由两个Cg E2三聚体和四个Cg E3二聚体组成。我们还发现Cg E1o主要以六聚体形式存在于溶液中,当与Cg E2-E3亚复合物混合时,很容易形成活性ODH复合物。我们的体外重构系统显示了Cg E1p‐和Cg分别ODH和PDH,的E1o依赖性抑制,积极地支持所述混合复合物的形成,其中两个CG E1P和CG E1o关联具有单CG E2-E3。在凝胶过滤色谱法,所有的亚单位CG ODH在相同的级分中洗脱,而CG E1P分别从洗脱CG E2-E3,提示的弱缔CG E1P与CG E2与比较CG E1o。这项研究揭示了C.杂合体的独特分子结构。 谷氨酸 紧凑型复合体将为确定异常混合复合体的整体结构提供优势。
更新日期:2020-10-17
中文翻译:

谷氨酸棒杆菌丙酮酸脱氢酶和2-氧戊二酸脱氢酶杂合体的体外重组和鉴定。
丙酮酸脱氢酶(PDH)和2-氧戊二酸脱氢酶(ODH)是中央碳代谢中的关键酶。在谷氨酸棒杆菌中,一种不寻常的杂合复合物,由Cg E1p(硫胺二磷酸依赖性丙酮酸脱氢酶,AceE),Cg E2(二氢脂酰胺乙酰转移酶,AceF),Cg E3(二氢脂酰胺脱氢酶,Lpd)和Cg E1o(硫胺素二磷酸依赖性2有人建议使用-oxoglutarate脱氢酶(OdhA)。在这里,我们阐明了C中的PDH-ODH杂合体。 谷氨酸可能由六份Cg组成E2的核心,与其他具有24个E2拷贝的微生物中的PDH和ODH相比,它非常紧凑。我们发现Cg E2在体外与Cg E3形成了稳定的复合物(Cg E2-E3亚复合物),假设由两个Cg E2三聚体和四个Cg E3二聚体组成。我们还发现Cg E1o主要以六聚体形式存在于溶液中,当与Cg E2-E3亚复合物混合时,很容易形成活性ODH复合物。我们的体外重构系统显示了Cg E1p‐和Cg分别ODH和PDH,的E1o依赖性抑制,积极地支持所述混合复合物的形成,其中两个CG E1P和CG E1o关联具有单CG E2-E3。在凝胶过滤色谱法,所有的亚单位CG ODH在相同的级分中洗脱,而CG E1P分别从洗脱CG E2-E3,提示的弱缔CG E1P与CG E2与比较CG E1o。这项研究揭示了C.杂合体的独特分子结构。 谷氨酸 紧凑型复合体将为确定异常混合复合体的整体结构提供优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号