当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Oxygen Reduction Reaction in Ca2+ -Containing DMSO: Reaction Mechanism, Electrode Surface Characterization, and Redox Mediation.
ChemSusChem ( IF 7.5 ) Pub Date : 2020-08-31 , DOI: 10.1002/cssc.202001605 Pawel Peter Bawol 1 , Philip Heinrich Reinsberg 1 , Andreas Koellisch-Mirbach 1 , Christoph Johannes Bondue 1 , Helmut Baltruschat 1
ChemSusChem ( IF 7.5 ) Pub Date : 2020-08-31 , DOI: 10.1002/cssc.202001605 Pawel Peter Bawol 1 , Philip Heinrich Reinsberg 1 , Andreas Koellisch-Mirbach 1 , Christoph Johannes Bondue 1 , Helmut Baltruschat 1
Affiliation
|
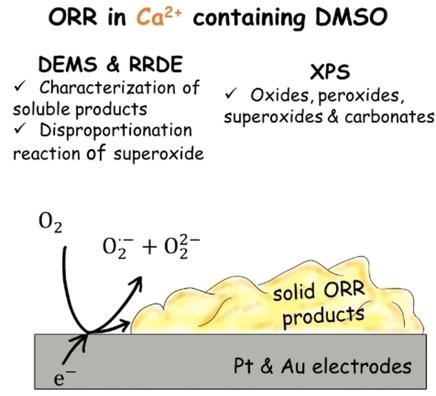
In this study the fundamental understanding of the underlying reactions of a possible Ca−O2 battery using a DMSO‐based electrolyte was strengthened. Employing the rotating ring disc electrode, a transition from a mixed process of O2− and O22− formation to an exclusive O2− formation at gold electrodes is observed. It is shown that in this system Ca‐superoxide and Ca‐peroxide are formed as soluble species. However, there is a strongly adsorbed layer of products of the oxygen reduction reaction (ORR) s on the electrode surface, which is blocking the electrode. Surprisingly the blockade is only a partial blockade for the formation of peroxide while the formation of superoxide is maintained. During an anodic sweep, the ORR product layer is stripped from the electrode surface. With X‐ray photoelectron spectroscopy (XPS) the deposited ORR products were shown to be Ca(O2)2, CaO2, and CaO as well as side‐reaction products such as CO32− and other oxygen‐containing carbon species. It is shown that the strongly attached layer on the electrocatalyst, that was partially blocking the electrode, could be adsorbed CaO. The disproportionation reaction of O2− in presence of Ca2+ was demonstrated via mass spectrometry. Finally, the ORR mediated by 2,5‐di‐tert‐1,4‐benzoquinone (DBBQ) was investigated by differential electrochemical mass spectrometry (DEMS) and XPS. Similar products as without DBBQ are deposited on the electrode surface. The analysis of the DEMS experiments shows that DBBQ− reduces O2 to O2− and O22−, whereas in the presence of DBBQ2− O22− is formed. The mechanism of the ORR with and without DBBQ is discussed.
中文翻译:

含 Ca2+ DMSO 中的氧还原反应:反应机理、电极表面表征和氧化还原介导。
在这项研究中,加强了对使用基于 DMSO 的电解质的可能 Ca−O 2电池的潜在反应的基本理解。使用旋转环盘电极,观察到金电极处从O 2 -和O 2 2-形成的混合过程到仅O 2 -形成的转变。结果表明,在该系统中,超氧化物钙和过氧化物钙以可溶性物质的形式形成。然而,电极表面有一层强烈吸附的氧还原反应(ORR)产物层,堵塞了电极。令人惊奇的是,该阻断仅部分阻断过氧化物的形成,同时维持超氧化物的形成。在阳极扫描过程中,ORR 产物层从电极表面剥离。通过 X 射线光电子能谱 (XPS),沉积的 ORR 产物显示为 Ca(O 2 ) 2、CaO 2和 CaO 以及副反应产物,例如 CO 3 2−和其他含氧碳物质。结果表明,电催化剂上的牢固附着层部分阻挡了电极,可以吸附 CaO。通过质谱法证明了Ca 2+存在下O 2 -的歧化反应。最后,通过差示电化学质谱(DEMS)和XPS研究了2,5-二叔-1,4-苯醌(DBBQ)介导的ORR 。与不含 DBBQ 的类似产物沉积在电极表面上。DEMS实验的分析表明,DBBQ -将O 2还原为O 2 -和O 2 2− ,而在DBBQ 2−存在的情况下形成O 2 2− 。讨论了有和没有 DBBQ 的 ORR 机制。
更新日期:2020-08-31
中文翻译:

含 Ca2+ DMSO 中的氧还原反应:反应机理、电极表面表征和氧化还原介导。
在这项研究中,加强了对使用基于 DMSO 的电解质的可能 Ca−O 2电池的潜在反应的基本理解。使用旋转环盘电极,观察到金电极处从O 2 -和O 2 2-形成的混合过程到仅O 2 -形成的转变。结果表明,在该系统中,超氧化物钙和过氧化物钙以可溶性物质的形式形成。然而,电极表面有一层强烈吸附的氧还原反应(ORR)产物层,堵塞了电极。令人惊奇的是,该阻断仅部分阻断过氧化物的形成,同时维持超氧化物的形成。在阳极扫描过程中,ORR 产物层从电极表面剥离。通过 X 射线光电子能谱 (XPS),沉积的 ORR 产物显示为 Ca(O 2 ) 2、CaO 2和 CaO 以及副反应产物,例如 CO 3 2−和其他含氧碳物质。结果表明,电催化剂上的牢固附着层部分阻挡了电极,可以吸附 CaO。通过质谱法证明了Ca 2+存在下O 2 -的歧化反应。最后,通过差示电化学质谱(DEMS)和XPS研究了2,5-二叔-1,4-苯醌(DBBQ)介导的ORR 。与不含 DBBQ 的类似产物沉积在电极表面上。DEMS实验的分析表明,DBBQ -将O 2还原为O 2 -和O 2 2− ,而在DBBQ 2−存在的情况下形成O 2 2− 。讨论了有和没有 DBBQ 的 ORR 机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号