Biomaterials Advances ( IF 5.5 ) Pub Date : 2020-08-31 , DOI: 10.1016/j.msec.2020.111471 Hongzhou Shen 1 , Jun Shi 1 , Yin Zhi 2 , Xiaoyan Yang 3 , Yuan Yuan 4 , Jiawen Si 2 , Steve G F Shen 1
|
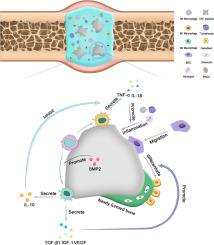
This study aimed to explore the in vitro and in vivo roles of macrophages in the osteogenesis stimulated by BMP2-CPC. In vitro, the alteration of macrophage polarization and cytokine secretion induced by BMP2-CPC or CPC was investigated. The influence of conditioned medium derived from BMP2-CPC- or CPC-stimulated macrophages on the migration and osteogenic differentiation of MSCs were evaluated. The in vivo relationship between macrophage polarization and osteogenesis was examined in a rabbit calvarial defect model. The in vitro results indicated that BMP2-CPC and CPC induced different patterns of macrophage polarization and subsequently resulted in distinct patterns of cytokine expression and secretion. Conditioned medium derived from BMP2-CPC- or CPC-stimulated macrophages both exhibited apparent osteogenic effect on MSCs. Notably, BMP2-CPC induced more M2-phenotype polarization and higher expression of anti-inflammatory cytokines and growth factors than did CPC, which led to the better osteogenic effect of conditioned medium derived from BMP2-CPC-stimulated macrophages. The rabbit calvarial defect model further confirmed that BMP2-CPC facilitated more bone regeneration than CPC did by enhancing M2-phenotype polarization in local macrophages and then alleviating inflammatory reaction. In conclusion, this study revealed that the favorable immunoregulatory property of BMP2-CPC contributed to the strong osteogenic capability of BMP2-CPC by modulating macrophage polarization.
中文翻译:

通过调节巨噬细胞极化改善 BMP2-CPC 刺激的体外和体内成骨作用
本研究旨在探讨巨噬细胞在 BMP2-CPC 刺激的成骨过程中的体外和体内作用。在体外,研究了 BMP2-CPC 或 CPC 诱导的巨噬细胞极化和细胞因子分泌的改变。评估了源自 BMP2-CPC 或 CPC 刺激的巨噬细胞的条件培养基对 MSC 迁移和成骨分化的影响。在兔颅骨缺损模型中检查了巨噬细胞极化与成骨之间的体内关系。体外结果表明 BMP2-CPC 和 CPC 诱导了不同的巨噬细胞极化模式,随后导致了不同的细胞因子表达和分泌模式。来自 BMP2-CPC 或 CPC 刺激的巨噬细胞的条件培养基均对 MSC 表现出明显的成骨作用。尤其,BMP2-CPC 比 CPC 诱导更多的 M2 表型极化和更高的抗炎细胞因子和生长因子表达,这导致源自 BMP2-CPC 刺激的巨噬细胞的条件培养基具有更好的成骨作用。兔颅骨缺损模型进一步证实,BMP2-CPC 通过增强局部巨噬细胞中的 M2 表型极化,然后减轻炎症反应,比 CPC 促进更多的骨再生。总之,这项研究表明,BMP2-CPC 有利的免疫调节特性通过调节巨噬细胞极化促进了 BMP2-CPC 的强大成骨能力。这导致源自 BMP2-CPC 刺激的巨噬细胞的条件培养基具有更好的成骨作用。兔颅骨缺损模型进一步证实,BMP2-CPC 通过增强局部巨噬细胞中的 M2 表型极化,然后减轻炎症反应,比 CPC 促进更多的骨再生。总之,这项研究表明,BMP2-CPC 有利的免疫调节特性通过调节巨噬细胞极化促进了 BMP2-CPC 的强大成骨能力。这导致源自 BMP2-CPC 刺激的巨噬细胞的条件培养基具有更好的成骨作用。兔颅骨缺损模型进一步证实,BMP2-CPC 通过增强局部巨噬细胞中的 M2 表型极化,然后减轻炎症反应,比 CPC 促进更多的骨再生。总之,这项研究表明,BMP2-CPC 有利的免疫调节特性通过调节巨噬细胞极化促进了 BMP2-CPC 的强大成骨能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号