Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-08-31 , DOI: 10.1016/j.bmcl.2020.127521 Qing Shi 1 , Zili Xiao 1 , Michael G Yang 1 , David Marcoux 1 , Robert J Cherney 1 , Shiuhang Yip 1 , Peng Li 1 , Dauh-Rurng Wu 1 , Carolyn A Weigelt 1 , John Sack 1 , Javed Khan 1 , Max Ruzanov 1 , Jinhong Wang 1 , Melissa Yarde 1 , Mary Ellen Cvijic 1 , Sha Li 1 , David J Shuster 1 , Jenny Xie 1 , Tara Sherry 1 , Mary Obermeier 1 , Aberra Fura 1 , Kevin Stefanski 1 , Georgia Cornelius 1 , Silvi Chacko 1 , Yue-Zhong Shu 1 , Purnima Khandelwal 1 , John Hynes 1 , Joseph A Tino 1 , Luisa Salter-Cid 1 , Rex Denton 1 , Qihong Zhao 1 , T G Murali Dhar 1
|
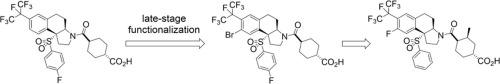
In order to rapidly develop C6 and C8 SAR of our reported tricyclic sulfone series of RORγt inverse agonists, a late-stage bromination was employed. Although not regioselective, the bromination protocol allowed us to explore new substitution patterns/vectors that otherwise would have to be incorporated at the very beginning of the synthesis. Based on the SAR obtained from this exercise, compound 15 bearing a C8 fluorine was developed as a very potent and selective RORγt inverse agonist. This analog’s in vitro profile, pharmacokinetic (PK) data and efficacy in an IL-23 induced mouse acanthosis model will be discussed.
中文翻译:

三环砜作为有效,选择性和有效的RORγt反向激动剂-使用后期功能化研究C6和C8 SAR。
为了快速开发我们报道的三环砜系列RORγt反向激动剂的C6和C8 SAR,采用了后期溴化。尽管不是区域选择性的,但溴化方案使我们能够探索新的取代模式/载体,否则必须在合成的一开始就将其引入。基于从该练习中获得的SAR,开发了具有C8氟的化合物15作为一种非常有效的选择性RORγt反向激动剂。将讨论该类似物的体外概况,药代动力学(PK)数据以及在IL-23诱导的小鼠棘皮病模型中的功效。







































 京公网安备 11010802027423号
京公网安备 11010802027423号