Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-08-31 , DOI: 10.1016/j.bmcl.2020.127518 Sumei Li 1 , Xiuhua Jia 2 , Hui Li 3 , Yilu Ye 4 , Xuesha Zhang 3 , Yongfeng Gao 3 , Guoqing Guo 1 , Shuwen Liu 4 , Gaopeng Song 3
|
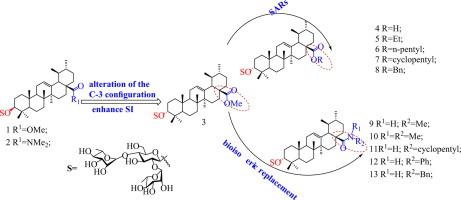
It is urgent to develop new antiviral agents due to the continuous emergence of drug-resistant strains of influenza virus. Our earlier studies have identified that certain pentacyclic triterpene saponins with 3-O-β-chacotriosyl residue are novel H5N1 virus entry inhibitors. In the present study, a series of C-28 modified 3-O-β-chacotriosyl epiursolic acid derivatives via conjugation with different kinds of sides were synthesized, of which anti-H5N1 activities in A549 cells were evaluated in vitro. Among them, 10 exhibited strongest anti-H5N1 potency at the low-micromole level without cytotoxicity, surpassing the potency of ribavirin. Further mechanism studies of the lead compound 10 based on HI, SPR and molecular modeling revealed that these new 3-epiursolic acid saponins could bind tightly to the viral envelope HA protein, thus blocking the invasion of H5N1 viruses into host cells.
中文翻译:

3-O-β-查考三糖基表熊果酸衍生物作为新型 H5N1 病毒进入抑制剂的结构辅助优化。
由于流感病毒耐药株的不断出现,迫切需要开发新的抗病毒药物。我们早期的研究发现,某些带有 3-O-β-chacottriosyl 残基的五环三萜皂苷是新型 H5N1 病毒侵入抑制剂。本研究合成了一系列通过不同侧面缀合的C-28修饰的3- O -β-查考三糖基表熊果酸衍生物,并在体外评价了其在A549细胞中的抗H5N1活性。其中, 10种在低微摩尔水平上表现出最强的抗H5N1效力,且无细胞毒性,超过了利巴韦林的效力。基于HI、SPR和分子模型对先导化合物10的进一步机制研究表明,这些新的3-表熊果酸皂苷可以与病毒包膜HA蛋白紧密结合,从而阻止H5N1病毒侵入宿主细胞。









































 京公网安备 11010802027423号
京公网安备 11010802027423号