当前位置:
X-MOL 学术
›
Gas Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Determination and Thermodynamic Modeling of Clathrate Hydrate Stability Conditions in Methane + Hydrogen Sulfide + Water System
Gas Science and Engineering Pub Date : 2020-11-01 , DOI: 10.1016/j.jngse.2020.103549 Mahnaz Aghajanloo , Zahra Taheri , Taraneh Jafari Behbahani , Amir H. Mohammadi , Mohammad Reza Ehsani , Hamed Heydarian
Gas Science and Engineering Pub Date : 2020-11-01 , DOI: 10.1016/j.jngse.2020.103549 Mahnaz Aghajanloo , Zahra Taheri , Taraneh Jafari Behbahani , Amir H. Mohammadi , Mohammad Reza Ehsani , Hamed Heydarian
|
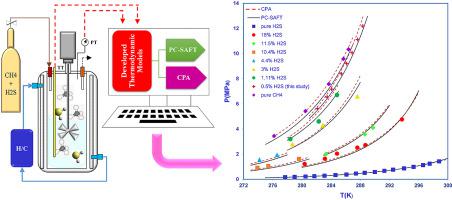
Abstract -In the petroleum industry, it is essential to comprehend the hydrate equilibrium qualifications in order to prevent or defer this phenomenon. However, the thermodynamic equilibrium information on hydrogen sulfide containing systems reported in the literature is limited and discrepant in comparison with other gases. In this communication, new experimental investigations of hydrate dissociation conditions of methane (0.995 mole fraction) + hydrogen sulfide (0.005 mole fraction) + water system which forms stable structure I were carried out in temperature and pressure ranges from 282.1 to 288.3 K and 5.63 to 12.19 MPa, respectively. The experiments were carried out by using the stepwise heating method with an isochoric pressure search procedure in a 100 cm3 autoclave. The apparatus and method validity were confirmed by comparison of the methane hydrate equilibrium data from the current study with some selected data from the literature. In this study, a thermodynamic model was developed to estimate the dissociation conditions of the binary CH4+H2S hydrate system. For this purpose, a systematic comparison is made between results from PC-SAFT (Perturbed-Chain Statistical Associating Fluid Theory) and CPA (Cubic Plus Association) equations of state (EoS). In the present work, PC-SAFT and CPA equations of state are utilized to model the gas/vapor and water phases and van der Waals Platteeuw (vdW-P) is used for hydrate phase modeling. In this regard, the binary interaction parameters in PC-SAFT/CPA EoS for the aforementioned system were tuned using literature data on H2S/CH4 solubility in water. Model outcomes show that both models are entirely trustworthy over an extensive range of temperatures (273 to 350 K), pressure (2 to 22 MPa) and all H2S concentrations. In addition, a comparison between experimental data, the models results and HWHydrate (version 1.1) predictions ascertain that the PC-SAFT is relatively precise than CPA in the binary hydrate system of CH4-H2S.
中文翻译:

甲烷+硫化氢+水体系中包合物水合物稳定性条件的实验测定和热力学建模
摘要 -在石油工业中,了解水合物平衡条件对于预防或延缓这种现象至关重要。然而,文献中报道的含硫化氢系统的热力学平衡信息有限,与其他气体相比存在差异。在本次通讯中,对甲烷(0.995 摩尔分数)+硫化氢(0.005 摩尔分数)+ 形成稳定结构 I 的水体系的水合物解离条件进行了新的实验研究,在温度和压力范围从 282.1 到 288.3 K 和 5.63 到分别为 12.19 兆帕。通过在 100 cm3 高压釜中使用等容压力搜索程序使用逐步加热方法进行实验。通过将当前研究中的甲烷水合物平衡数据与一些从文献中选择的数据进行比较,证实了仪器和方法的有效性。在这项研究中,开发了一个热力学模型来估计二元 CH4+H2S 水合物系统的解离条件。为此,对 PC-SAFT(扰动链统计关联流体理论)和 CPA(立方加关联)状态方程 (EoS) 的结果进行了系统比较。在目前的工作中,PC-SAFT 和 CPA 状态方程用于模拟气/汽和水相,范德华普拉特 (vdW-P) 用于水合物相建模。在这方面,使用有关 H2S/CH4 在水中溶解度的文献数据对上述系统的 PC-SAFT/CPA EoS 中的二元相互作用参数进行了调整。模型结果表明,这两种模型在广泛的温度(273 至 350 K)、压力(2 至 22 MPa)和所有 H2S 浓度范围内都完全值得信赖。此外,通过比较实验数据、模型结果和 HWHydrate(版本 1.1)预测,PC-SAFT 在 CH4-H2S 二元水合物系统中比 CPA 相对精确。
更新日期:2020-11-01
中文翻译:

甲烷+硫化氢+水体系中包合物水合物稳定性条件的实验测定和热力学建模
摘要 -在石油工业中,了解水合物平衡条件对于预防或延缓这种现象至关重要。然而,文献中报道的含硫化氢系统的热力学平衡信息有限,与其他气体相比存在差异。在本次通讯中,对甲烷(0.995 摩尔分数)+硫化氢(0.005 摩尔分数)+ 形成稳定结构 I 的水体系的水合物解离条件进行了新的实验研究,在温度和压力范围从 282.1 到 288.3 K 和 5.63 到分别为 12.19 兆帕。通过在 100 cm3 高压釜中使用等容压力搜索程序使用逐步加热方法进行实验。通过将当前研究中的甲烷水合物平衡数据与一些从文献中选择的数据进行比较,证实了仪器和方法的有效性。在这项研究中,开发了一个热力学模型来估计二元 CH4+H2S 水合物系统的解离条件。为此,对 PC-SAFT(扰动链统计关联流体理论)和 CPA(立方加关联)状态方程 (EoS) 的结果进行了系统比较。在目前的工作中,PC-SAFT 和 CPA 状态方程用于模拟气/汽和水相,范德华普拉特 (vdW-P) 用于水合物相建模。在这方面,使用有关 H2S/CH4 在水中溶解度的文献数据对上述系统的 PC-SAFT/CPA EoS 中的二元相互作用参数进行了调整。模型结果表明,这两种模型在广泛的温度(273 至 350 K)、压力(2 至 22 MPa)和所有 H2S 浓度范围内都完全值得信赖。此外,通过比较实验数据、模型结果和 HWHydrate(版本 1.1)预测,PC-SAFT 在 CH4-H2S 二元水合物系统中比 CPA 相对精确。











































 京公网安备 11010802027423号
京公网安备 11010802027423号