Energy Storage Materials ( IF 18.9 ) Pub Date : 2020-08-30 , DOI: 10.1016/j.ensm.2020.08.016 Feng Guo , Zheng Huang , Mingyong Wang , Wei-Li Song , Aijing Lv , Xue Han , Jiguo Tu , Shuqiang Jiao
|
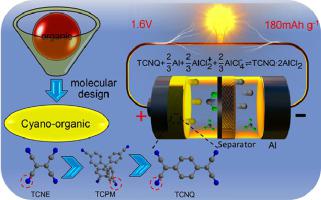
Rechargeable aluminum batteries, owing to the abundant Al resources and high safety guarantee, have been exploited as the ideal power sources for large-scale energy storage. However, the application of aluminum batteries is still restricted by the unsatisfactory positive electrodes due to low capacity, electrode variation or poor cycle stability of inorganic materials. As an alternative route for addressing the critical issues, multi-cyano organic molecules are proposed as the positive electrode materials for stable rechargeable aluminum batteries. With the first-principle calculation of the electron distribution, molecular energy levels and theoretical specific capacity, the results suggest that the electron-deficient cyano groups in organic molecules can reversibly coordinate/dissociate with positively charged Al-complex ions (i.e. AlCl2+). According to the molecule design and experiment results, tetracyanoquinodimethane (TCNQ) with high conductivity is identified as the optimized cyano-organic positive electrode material. The as-assembled cell delivers a high specific capacity of 180 mAh g−1 at first cycle and a discharge plateau ~1.6 V, along with long-term operation beyond 2000 cycles and coulombic efficiency of ~100%. The design principle here opens a new platform to utilize stable cyano-organic molecules as positive electrode materials for high-energy-density aluminum batteries, offering opportunities to design and exploit high-performance rechargeable Al-organic batteries.
中文翻译:

活性氰基配位可充电铝电池的AlCl 2 +阳离子
可充电铝电池由于其丰富的铝资源和高安全性保证,已被用作大规模储能的理想电源。然而,由于低容量,电极变化或无机材料的循环稳定性差,铝电池的应用仍然受到不能令人满意的正极的限制。作为解决关键问题的替代途径,提出了多氰基有机分子作为稳定可再充电铝电池的正极材料。通过对电子分布,分子能级和理论比容量的第一性原理计算,结果表明有机分子中缺电子的氰基可以与带正电的Al络合物离子(即AlCl)可逆地配位/离解。2 +)。根据分子设计和实验结果,高电导率的四氰基喹二甲烷(TCNQ)被确定为最佳的氰基有机正电极材料。组装后的电池在第一个循环中提供180 mAh g -1的高比容量,并且放电平稳在〜1.6 V,并且在2000次循环后可以长期运行,库仑效率达到〜100%。这里的设计原则为使用稳定的氰基有机分子作为高能量密度铝电池的正极材料开辟了一个新平台,为设计和开发高性能可充电铝有机电池提供了机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号