Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-08-29 , DOI: 10.1016/j.bmc.2020.115736 Chenghu Xie 1 , Yongmei Cui 1 , Lanlan Li 2 , Minmin Zhang 2 , Hongchun Liu 2 , Haixia Lin 1
|
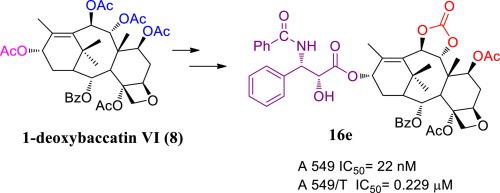
A series of C-7, C-9 and C-10 modified taxane analogues were synthesized and their in vitro anticancer activities against three human cancer cell lines: A-549 (human lung cancer cell line), MDA-MB-231 (human breast cancer cell line), A-549/T (human lung cancer resistant cell line) were studied. The novel 1-deoxybaccatin VI derivatives modified with carbonate group at C-9 and C-10 positions enable the behavior of these compounds to be evidently distinct from other similar compounds. The strong cytotoxicity in the three cell lines, especially in drug-resistant cell line, showed by the newly synthesized taxane analogues indicated them as potential lead compounds for anticancer drug design.
中文翻译:

1-脱氧浆果赤霉素VI合成C-7,C-9和C-10修饰的紫杉烷类似物及其生物学活性
合成了一系列经C-7,C-9和C-10修饰的紫杉烷类似物,它们对三种人类癌细胞系:A-549(人类肺癌细胞系),MDA-MB-231(人类)具有体外抗癌活性。乳腺癌细胞系),A-549 / T(抗人肺癌细胞系)进行了研究。在C-9和C-10位置用碳酸酯基修饰的新型1-脱氧浆果赤霉素VI衍生物使这些化合物的行为与其他类似化合物明显不同。新合成的紫杉烷类似物在这三种细胞系中,特别是在耐药细胞系中具有很强的细胞毒性,表明它们是抗癌药物设计的潜在先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号