当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct electrochemical synthesis of oxygenates from ethane using phosphate-based electrolysis cells.
Chemical Communications ( IF 4.9 ) Pub Date : 2020-08-28 , DOI: 10.1039/d0cc05111j Yusuke Honda 1 , Naoya Fujiwara 1 , Shohei Tada 2 , Yasukazu Kobayashi 3 , Shigeo Ted Oyama 4 , Ryuji Kikuchi 1
Chemical Communications ( IF 4.9 ) Pub Date : 2020-08-28 , DOI: 10.1039/d0cc05111j Yusuke Honda 1 , Naoya Fujiwara 1 , Shohei Tada 2 , Yasukazu Kobayashi 3 , Shigeo Ted Oyama 4 , Ryuji Kikuchi 1
Affiliation
|
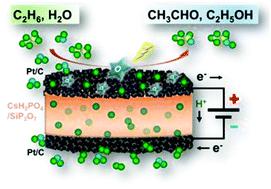
Ethane was converted directly to acetaldehyde and ethanol by partial oxidation at 220 °C and ambient pressure using an electrolysis cell with a proton-conducting electrolyte, CsH2PO4/SiP2O7, and Pt/C electrodes. The ethane conversion and the selectivity to the products increased with the voltage applied to the cell. It was found that O species generated by water electrolysis functioned as a favorable oxidant for partial oxidation of ethane on the Pt/C anode at intermediate temperatures. The production rates of acetaldehyde and ethanol recorded in this study were significantly higher than those in preceding reports.
中文翻译:

使用基于磷酸盐的电解池直接从乙烷中电化学合成含氧化合物。
通过使用带有质子传导电解质,CsH 2 PO 4 / SiP 2 O 7和Pt / C电极的电解槽,在220°C和环境压力下进行部分氧化,将乙烷直接转化为乙醛和乙醇。乙烷转化率和产物选择性随施加到电解池的电压而增加。已经发现,在中间温度下,水电解产生的O物种起着使Pt / C阳极上的乙烷部分氧化的有利氧化剂的作用。这项研究中记录的乙醛和乙醇的生产率显着高于以前的报告。
更新日期:2020-09-24
中文翻译:

使用基于磷酸盐的电解池直接从乙烷中电化学合成含氧化合物。
通过使用带有质子传导电解质,CsH 2 PO 4 / SiP 2 O 7和Pt / C电极的电解槽,在220°C和环境压力下进行部分氧化,将乙烷直接转化为乙醛和乙醇。乙烷转化率和产物选择性随施加到电解池的电压而增加。已经发现,在中间温度下,水电解产生的O物种起着使Pt / C阳极上的乙烷部分氧化的有利氧化剂的作用。这项研究中记录的乙醛和乙醇的生产率显着高于以前的报告。



























 京公网安备 11010802027423号
京公网安备 11010802027423号