当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Dearomatization/Rearomatization of 2-Nitroindoles to Access 3-Indolyl-3'-Aryl-/Alkyloxindoles: Application in the Formal Synthesis of Cyclotryptamine Alkaloids.
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-28 , DOI: 10.1021/acs.orglett.0c02350 Wei-Cheng Yuan 1 , Xiao-Jian Zhou 2 , Jian-Qiang Zhao 1 , Yong-Zheng Chen 2 , Yong You 1 , Zhen-Hua Wang 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-28 , DOI: 10.1021/acs.orglett.0c02350 Wei-Cheng Yuan 1 , Xiao-Jian Zhou 2 , Jian-Qiang Zhao 1 , Yong-Zheng Chen 2 , Yong You 1 , Zhen-Hua Wang 1
Affiliation
|
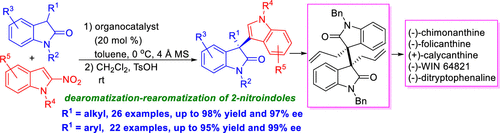
The first catalytic enantioselective dearomatization/rearomatization of 2-nitroindoles triggered by the Michael addition of 3-monosubstituted oxindoles was established. A wide range of 3-indolyl-3′-alkyloxindoles (up to 99% yield, 97% ee) and 3-indolyl-3′-aryloxindoles (up to 95% yield, 99% ee) were obtained by using an organocatalyst. This method provides an unprecedented strategy to access structurally diverse 3,3′-disubstituted oxindoles bearing a sterically congested triaryl-containing all-carbon quaternary stereocenter. The utility of this approach was verified by the formal synthesis of cyclotryptamine alkaloids.
中文翻译:

2-硝基吲哚的催化对映选择性脱芳香化/再芳香化以获取3-Indolyl-3'-Aryl- / Alkyloxindoles:在环草胺生物碱的形式合成中的应用。
建立了由3-单取代的羟吲哚的迈克尔加成引发的2-硝基吲哚的第一催化对映选择性脱芳香化/再芳香化。通过使用有机催化剂,获得了各种各样的3-吲哚基-3'-烷基氧吲哚(产率高达99%,ee为97%)和3-吲哚基-3'-芳基羟吲哚(产率高达95%,ee为99%)。该方法提供了一种空前的策略,可访问带有空间拥挤的含三芳基全碳四元立体中心的结构多样的3,3'-二取代的羟吲哚。通过环色胺生物碱的正式合成,验证了该方法的实用性。
更新日期:2020-09-20
中文翻译:

2-硝基吲哚的催化对映选择性脱芳香化/再芳香化以获取3-Indolyl-3'-Aryl- / Alkyloxindoles:在环草胺生物碱的形式合成中的应用。
建立了由3-单取代的羟吲哚的迈克尔加成引发的2-硝基吲哚的第一催化对映选择性脱芳香化/再芳香化。通过使用有机催化剂,获得了各种各样的3-吲哚基-3'-烷基氧吲哚(产率高达99%,ee为97%)和3-吲哚基-3'-芳基羟吲哚(产率高达95%,ee为99%)。该方法提供了一种空前的策略,可访问带有空间拥挤的含三芳基全碳四元立体中心的结构多样的3,3'-二取代的羟吲哚。通过环色胺生物碱的正式合成,验证了该方法的实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号