Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Mechanism of the Reduction of Ozone on Graphite.
Langmuir ( IF 3.7 ) Pub Date : 2020-08-28 , DOI: 10.1021/acs.langmuir.0c01372 Eduardo Humeres , Karen M. de Castro , Nito Angelo Debacher , Regina de F. P. M. Moreira
Langmuir ( IF 3.7 ) Pub Date : 2020-08-28 , DOI: 10.1021/acs.langmuir.0c01372 Eduardo Humeres , Karen M. de Castro , Nito Angelo Debacher , Regina de F. P. M. Moreira
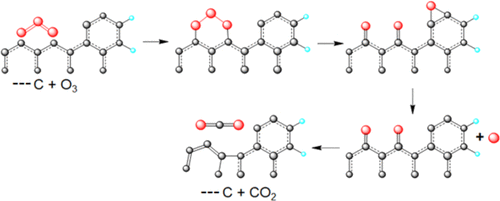
|
The kinetics of the ozonation of graphite with different particle sizes (106 μm, G106; 6.20 μm, G6.2) was studied at several temperatures under a flow of O3 diluted in O2. The reaction was first-order with respect to graphite and to the consumption of ozone. X-ray photoelectron spectrum (XPS) showed that the reactions occurring in the solid under steady-state conditions maintain the original stoichiometry, as predicted by the postulated mechanism for SO2. The deoxygenation reaction occurred along with the ozonation reaction at 100 °C. The rate of oxygen elimination in the flow system has the same rate-determining kinetic barrier as ozone insertion. Ozonation and deoxygenation reactions are sequentially related. Ozonation occurs with the insertion of O3, forming a 1,2,3-trioxolane followed by an oxygen transfer that produces a peroxide valence tautomer in equilibrium with 1,3-dicarbonyl, [peroxide ↔ dicarbonyl], and an oxirene that eliminates atomic oxygen. The decarboxylation reaction was studied at 600 °C from the ozonated G106 (ΔG≠ = 83.60 ± 0.08 kcal·mol–1). Total decarboxylation at 600 °C matched the number of moles of CO2 removed and the oxygen content after ozonation, showing that the reduction of ozone on graphite was essentially a clean reduction with no secondary oxidations. When ozonized graphite was heated to 600 °C, only [peroxide ↔ dicarbonyl] species remained in the matrix. The peroxide tautomer isomerized to dioxirane and eliminated CO2 as a dioxicarbene. Total deoxygenation of decarboxylated graphite G106 was obtained by pyrolysis. There was residual oxygen that arose from the atomic oxygen eliminated from the oxirene, intercalated in graphite layers, and formed basal epoxy groups. Also, incoming O atoms reacted with the intercalated O atoms to produce O2 molecules. Thermal annealing deintercalated molecular oxygen (600–900 °C).
中文翻译:

石墨上臭氧还原的反应机理。
在几个温度下,在O 2中稀释的O 3流动下,研究了不同粒径(106μm,G 106; 6.20μm,G 6.2)的石墨的臭氧氧化动力学。就石墨和臭氧消耗而言,该反应是一级反应。X射线光电子能谱(XPS)表明,在固态条件下在固体中发生的反应保持了最初的化学计量,这是由SO 2的推测机理预测的。在100°C下,脱氧反应与臭氧化反应同时发生。流动系统中氧气的消除速率具有与臭氧插入相同的速率决定动力学障碍。臭氧化和脱氧反应是顺序相关的。臭氧化是随着O 3的插入而发生的,形成1,2,3-三氧戊环,随后进行氧气转移,产生与1,3-二羰基,[过氧化物↔二羰基]和消除原子的环氧乙烷平衡的过氧化物价互变异构体。氧。研究了在600°C下从臭氧化的G 106进行的脱羧反应(ΔG ≠ = 83.60±0.08 kcal·mol –1)。600°C时的总脱羧量与CO 2的摩尔数匹配去除臭氧层后的氧含量,这表明在石墨上臭氧的还原基本上是干净的还原,没有二次氧化。当将臭氧化的石墨加热至600°C时,基体中仅残留[过氧化物↔二羰基]物质。过氧化物互变异构体异构化成二环氧乙烷并消除了作为二氧卡宾的CO 2。通过热解获得脱羧石墨G 106的总脱氧。从环氧乙烷中除去的原子氧中产生了残留的氧,该氧嵌入石墨层中并形成基础环氧基。而且,进入的O原子与插入的O原子反应以产生O 2分子。热退火脱氧分子氧(600–900°C)。
更新日期:2020-09-29
中文翻译:

石墨上臭氧还原的反应机理。
在几个温度下,在O 2中稀释的O 3流动下,研究了不同粒径(106μm,G 106; 6.20μm,G 6.2)的石墨的臭氧氧化动力学。就石墨和臭氧消耗而言,该反应是一级反应。X射线光电子能谱(XPS)表明,在固态条件下在固体中发生的反应保持了最初的化学计量,这是由SO 2的推测机理预测的。在100°C下,脱氧反应与臭氧化反应同时发生。流动系统中氧气的消除速率具有与臭氧插入相同的速率决定动力学障碍。臭氧化和脱氧反应是顺序相关的。臭氧化是随着O 3的插入而发生的,形成1,2,3-三氧戊环,随后进行氧气转移,产生与1,3-二羰基,[过氧化物↔二羰基]和消除原子的环氧乙烷平衡的过氧化物价互变异构体。氧。研究了在600°C下从臭氧化的G 106进行的脱羧反应(ΔG ≠ = 83.60±0.08 kcal·mol –1)。600°C时的总脱羧量与CO 2的摩尔数匹配去除臭氧层后的氧含量,这表明在石墨上臭氧的还原基本上是干净的还原,没有二次氧化。当将臭氧化的石墨加热至600°C时,基体中仅残留[过氧化物↔二羰基]物质。过氧化物互变异构体异构化成二环氧乙烷并消除了作为二氧卡宾的CO 2。通过热解获得脱羧石墨G 106的总脱氧。从环氧乙烷中除去的原子氧中产生了残留的氧,该氧嵌入石墨层中并形成基础环氧基。而且,进入的O原子与插入的O原子反应以产生O 2分子。热退火脱氧分子氧(600–900°C)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号