当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arrangements of proteins at reconstituted synaptic vesicle fusion sites depend on membrane separation.
FEBS Letters ( IF 3.0 ) Pub Date : 2020-09-12 , DOI: 10.1002/1873-3468.13916 Lucy Ginger 1 , Joerg Malsam 2 , Andreas F-P Sonnen 3 , Dustin Morado 1 , Andrea Scheutzow 2 , Thomas H Söllner 2 , John A G Briggs 1, 3
FEBS Letters ( IF 3.0 ) Pub Date : 2020-09-12 , DOI: 10.1002/1873-3468.13916 Lucy Ginger 1 , Joerg Malsam 2 , Andreas F-P Sonnen 3 , Dustin Morado 1 , Andrea Scheutzow 2 , Thomas H Söllner 2 , John A G Briggs 1, 3
Affiliation
|
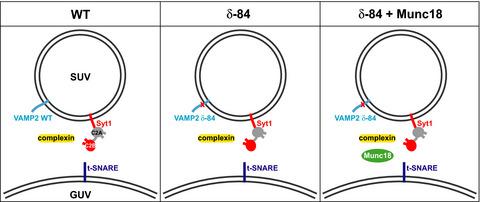
Synaptic vesicle proteins, including N‐ethylmaleimide‐sensitive factor attachment protein receptors (SNAREs), Synaptotagmin‐1 and Complexin, are responsible for controlling the synchronised fusion of synaptic vesicles with the presynaptic plasma membrane in response to elevated cytosolic calcium levels. A range of structures of SNAREs and their regulatory proteins have been elucidated, but the exact organisation of these proteins at synaptic junction membranes remains elusive. Here, we have used cryoelectron tomography to investigate the arrangement of synaptic proteins in an in vitro reconstituted fusion system. We found that the separation between vesicle and target membranes strongly correlates with the organisation of protein complexes at junctions. At larger membrane separations, protein complexes assume a ‘clustered’ distribution at the docking site, inducing a protrusion in the target membrane. As the membrane separation decreases, protein complexes become displaced radially outwards and assume a ‘ring‐like’ arrangement. Our findings indicate that docked vesicles can possess a wide range of protein complex numbers and be heterogeneous in their protein arrangements.
中文翻译:

重组突触囊泡融合位点的蛋白质排列取决于膜分离。
突触囊泡蛋白,包括 N-乙基马来酰亚胺敏感因子附着蛋白受体 (SNARE)、Synaptotagmin-1 和 Complexin,负责控制突触囊泡与突触前质膜的同步融合,以响应细胞溶质钙水平升高。SNARE 及其调节蛋白的一系列结构已被阐明,但这些蛋白质在突触连接膜上的确切组织仍然难以捉摸。在这里,我们使用冷冻电子断层扫描来研究体外重组融合系统中突触蛋白的排列。我们发现囊泡和靶膜之间的分离与连接处蛋白质复合物的组织密切相关。在较大的膜分离中,蛋白质复合物在对接位点呈“簇状”分布,诱导目标膜突出。随着膜分离度的降低,蛋白质复合物径向向外移动并呈现“环状”排列。我们的研究结果表明,对接的囊泡可以拥有广泛的蛋白质复合数,并且它们的蛋白质排列是异质的。
更新日期:2020-09-12
中文翻译:

重组突触囊泡融合位点的蛋白质排列取决于膜分离。
突触囊泡蛋白,包括 N-乙基马来酰亚胺敏感因子附着蛋白受体 (SNARE)、Synaptotagmin-1 和 Complexin,负责控制突触囊泡与突触前质膜的同步融合,以响应细胞溶质钙水平升高。SNARE 及其调节蛋白的一系列结构已被阐明,但这些蛋白质在突触连接膜上的确切组织仍然难以捉摸。在这里,我们使用冷冻电子断层扫描来研究体外重组融合系统中突触蛋白的排列。我们发现囊泡和靶膜之间的分离与连接处蛋白质复合物的组织密切相关。在较大的膜分离中,蛋白质复合物在对接位点呈“簇状”分布,诱导目标膜突出。随着膜分离度的降低,蛋白质复合物径向向外移动并呈现“环状”排列。我们的研究结果表明,对接的囊泡可以拥有广泛的蛋白质复合数,并且它们的蛋白质排列是异质的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号