当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
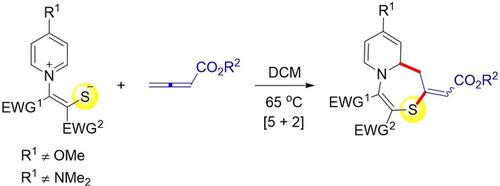
Synthesis of Pyridothiazepines via a 1,5‐Dipolar Cycloaddition Reaction between Pyridinium 1,4‐Zwitterionic Thiolates and Activated Allenes
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-08-28 , DOI: 10.1002/adsc.202000655 Bin Cheng 1, 2 , Xinping Zhang 1, 2 , Hui Li 1, 2 , Yixuan He 1, 2 , Yun Li 1 , Haiyan Sun 2 , Taimin Wang 2 , Hongbin Zhai 2, 3
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-08-28 , DOI: 10.1002/adsc.202000655 Bin Cheng 1, 2 , Xinping Zhang 1, 2 , Hui Li 1, 2 , Yixuan He 1, 2 , Yun Li 1 , Haiyan Sun 2 , Taimin Wang 2 , Hongbin Zhai 2, 3
Affiliation
|
The synthesis of pyridothiazepines has been achieved via a 1,5‐dipolar cycloaddition reaction between pyridinium 1,4‐zwitterionic thiolates and activated allenes conducted under thermal conditions. Meanwhile, a ring‐contraction reaction of the resulting pyridothiazepine derivatives via the extrusion of 3‐thioxoacrylates has also been described.
中文翻译:

通过1,4-两性离子硫代吡啶盐与活化的烯类化合物之间的1,5-偶极环加成反应合成吡啶并氮杂卓
吡啶硫并氮杂卓类化合物的合成是通过在热条件下进行的吡啶鎓1,4-两性离子硫醇盐和活化的丙二烯之间的1,5-偶极环加成反应实现的。同时,还描述了通过3-硫代丙烯酸酯的挤出反应生成的吡啶硫并ze庚因衍生物的环收缩反应。
更新日期:2020-11-04
中文翻译:

通过1,4-两性离子硫代吡啶盐与活化的烯类化合物之间的1,5-偶极环加成反应合成吡啶并氮杂卓
吡啶硫并氮杂卓类化合物的合成是通过在热条件下进行的吡啶鎓1,4-两性离子硫醇盐和活化的丙二烯之间的1,5-偶极环加成反应实现的。同时,还描述了通过3-硫代丙烯酸酯的挤出反应生成的吡啶硫并ze庚因衍生物的环收缩反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号