Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2020-08-29 , DOI: 10.1016/j.jorganchem.2020.121498 Diana V. Aleksanyan , Svetlana G. Churusova , Valentina V. Brunova , Ekaterina Yu. Rybalkina , Olga Yu. Susova , Alexander S. Peregudov , Zinaida S. Klemenkova , Gleb L. Denisov , Vladimir A. Kozlov
|
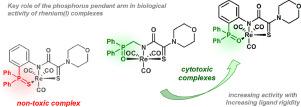
ortho-Phosphorylated aniline and its thio analog were used as key precursors for the synthesis of new multidentate ligands based on functionalized monothiooxamides. These compounds readily formed pincer-type complexes in reactions with Re(CO)5Br upon addition of a base. The realization of a tridentate monoanionic coordination of the functionalized monothiooxamide ligands in the resulting complexes through the oxygen or sulfur atom of the ancillary P=X donor group, the nitrogen atom of the deprotonated amide unit, and the sulfur atom of the thioamide moiety was unambiguously confirmed by the multinuclear NMR and IR spectroscopic data as well as X-ray diffraction. Investigation of the cytotoxic properties of these complexes against several human cancer cell lines revealed the high potential of the P(O)-substituted derivative. For comparison, the related complex with the more flexible phosphoryl pendant arm was prepared in an analogous manner and tested for cytotoxic activity. Although this compound also demonstrated remarkable cytotoxic effects on the cancer cell lines explored, it was slightly inferior to its analog with the more rigid phosphorus(V) coordination arm. The cytotoxic properties of the rhenium complexes were shown to be associated mainly with the coordination by Re(I) ions, since the free monothiooxamide ligands almost do not inhibit or even stimulate proliferation of the cancer cells. All this renders the creation of new potential anticancer agents based on Re(I) pincer complexes of functionalized ortho-phosphorylanilides very promising.
中文翻译:

具有(硫代)磷酰基侧基臂的N-金属化rh(I)钳形配合物的合成,表征和细胞毒活性
邻磷酸化的苯胺及其硫代类似物被用作基于功能化的单硫代乙酰胺合成新的多齿配体的关键前体。这些化合物在与Re(CO)5反应时容易形成钳型复合物加上碱即可。通过辅助P = X供体基团的氧或硫原子,去质子化酰胺单元的氮原子和硫酰胺部分的硫原子,在所得配合物中实现官能化的单硫代乙酰胺配体的三齿单阴离子配位由多核NMR和IR光谱数据以及X射线衍射证实。这些复合物对几种人类癌细胞系的细胞毒性研究表明,P(O)取代的衍生物具有很高的潜力。为了进行比较,以类似的方式制备了具有更柔性的磷酸基侧基臂的相关复合物,并测试了其细胞毒性活性。尽管该化合物还对探索的癌细胞系表现出了显着的细胞毒性作用,它的刚性稍差于其磷(V)配位臂。the配合物的细胞毒性被证明主要与Re(I)离子的配位有关,因为游离的单硫代草酰胺配体几乎不抑制甚至刺激癌细胞的增殖。所有这些都使得基于功能化的Re(I)钳复合物的新型潜在抗癌药的产生成为可能。邻位磷腈非常有前景。



























 京公网安备 11010802027423号
京公网安备 11010802027423号