当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron doped nanocomposites based efficient catalyst for hydrogen production and reduction of organic pollutant
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.colsurfa.2020.125502 Sher Bahadar Khan , Tahseen Kamal , Abdullah M. Asiri , Esraa M. Bakhsh
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.colsurfa.2020.125502 Sher Bahadar Khan , Tahseen Kamal , Abdullah M. Asiri , Esraa M. Bakhsh
|
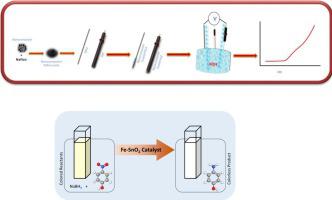
Abstract Iron doped nanocomposites based effective material were prepared for catalytic reduction of an organic pollutant and hydrogen production. Electrochemical production of hydrogen based on water splitting has been deliberated as an auspicious approach, however, the lethargic anodic oxygen evolution reaction (OER) restricts the effectiveness of water splitting. Also, active catalysts for catalytic reduction of organic pollutants are also highly required for the catalytic removal of organic pollutants. Therefore, in this manuscript, we report iron doped metal oxide nanocomposites (Fe2O3-ZnO and Fe-SnO2) as multifunctional catalysts for OER, hydrogen evolution reaction (HER) as well as catalytic reduction of organic pollutants. Therefore, efficient nano-catalyst based on iron oxide doped tin oxides and iron doped zinc oxide with long-term stability and efficient hydrogen generation and removal of organic pollutants has been developed. The catalytic efficiency of the developed materials was evaluated toward producing hydrogen and removing organic pollutants. In HER, it was found that Fe2O3-ZnO and Fe-SnO2 needs a low over potential to produce 10 mA cm−2 in 0.5 M KOH and 0.5 M H2SO4. Similarly, in OER, 600 mV is needed to drive the current density of 20 mA cm−2 in 1.0 M KOH using Fe2O3-ZnO. Fe2O3-ZnO illustrates lower Tafel slopes of 64.3 mV dec-1 and 96.0 mV dec-1 in HER using 0.5 M KOH and 0.5 M H2SO4, respectively, while in OER, the Tafel slope is 76.5 mV dec-1 using 1.0 M KOH. The catalytic efficiency of Fe2O3-ZnO and Fe-SnO2 was also evaluated for the removal of 4-nitrophenol and among them, Fe-SnO2 displayed good catalytic efficiency toward 4-nitrophenol reduction. 4-Nitrophenol was totally reduced in less than 5 min using Fe-SnO2 as a catalyst.
中文翻译:

基于铁掺杂纳米复合材料的高效制氢和有机污染物还原催化剂
摘要 制备了基于铁掺杂纳米复合材料的有效材料,用于催化还原有机污染物和制氢。基于水分解的电化学制氢被认为是一种吉祥的方法,然而,昏昏欲睡的阳极析氧反应 (OER) 限制了水分解的有效性。此外,催化去除有机污染物也非常需要用于催化还原有机污染物的活性催化剂。因此,在本手稿中,我们报道了铁掺杂金属氧化物纳米复合材料(Fe2O3-ZnO 和 Fe-SnO2)作为 OER、析氢反应 (HER) 以及催化还原有机污染物的多功能催化剂。所以,开发了基于氧化铁掺杂氧化锡和铁掺杂氧化锌的高效纳米催化剂,具有长期稳定性和高效的制氢和去除有机污染物的能力。对所开发材料的催化效率进行了评估,以产生氢气和去除有机污染物。在 HER 中,发现 Fe2O3-ZnO 和 Fe-SnO2 需要低过电位才能在 0.5 M KOH 和 0.5 M H2SO4 中产生 10 mA cm-2。类似地,在 OER 中,使用 Fe2O3-ZnO 在 1.0 M KOH 中驱动 20 mA cm-2 的电流密度需要 600 mV。Fe2O3-ZnO 在使用 0.5 M KOH 和 0.5 M H2SO4 的 HER 中显示较低的 Tafel 斜率,分别为 64.3 mV dec-1 和 96.0 mV dec-1,而在 OER 中,使用 1.0 M KOH 时的 Tafel 斜率为 76.5 mV dec-1。还评估了 Fe2O3-ZnO 和 Fe-SnO2 对 4-硝基苯酚去除的催化效率,其中 Fe-SnO2 对 4-硝基苯酚的还原显示出良好的催化效率。使用 Fe-SnO2 作为催化剂,4-硝基苯酚在不到 5 分钟的时间内被完全还原。
更新日期:2021-01-01
中文翻译:

基于铁掺杂纳米复合材料的高效制氢和有机污染物还原催化剂
摘要 制备了基于铁掺杂纳米复合材料的有效材料,用于催化还原有机污染物和制氢。基于水分解的电化学制氢被认为是一种吉祥的方法,然而,昏昏欲睡的阳极析氧反应 (OER) 限制了水分解的有效性。此外,催化去除有机污染物也非常需要用于催化还原有机污染物的活性催化剂。因此,在本手稿中,我们报道了铁掺杂金属氧化物纳米复合材料(Fe2O3-ZnO 和 Fe-SnO2)作为 OER、析氢反应 (HER) 以及催化还原有机污染物的多功能催化剂。所以,开发了基于氧化铁掺杂氧化锡和铁掺杂氧化锌的高效纳米催化剂,具有长期稳定性和高效的制氢和去除有机污染物的能力。对所开发材料的催化效率进行了评估,以产生氢气和去除有机污染物。在 HER 中,发现 Fe2O3-ZnO 和 Fe-SnO2 需要低过电位才能在 0.5 M KOH 和 0.5 M H2SO4 中产生 10 mA cm-2。类似地,在 OER 中,使用 Fe2O3-ZnO 在 1.0 M KOH 中驱动 20 mA cm-2 的电流密度需要 600 mV。Fe2O3-ZnO 在使用 0.5 M KOH 和 0.5 M H2SO4 的 HER 中显示较低的 Tafel 斜率,分别为 64.3 mV dec-1 和 96.0 mV dec-1,而在 OER 中,使用 1.0 M KOH 时的 Tafel 斜率为 76.5 mV dec-1。还评估了 Fe2O3-ZnO 和 Fe-SnO2 对 4-硝基苯酚去除的催化效率,其中 Fe-SnO2 对 4-硝基苯酚的还原显示出良好的催化效率。使用 Fe-SnO2 作为催化剂,4-硝基苯酚在不到 5 分钟的时间内被完全还原。











































 京公网安备 11010802027423号
京公网安备 11010802027423号