Chemical Engineering Research and Design ( IF 3.7 ) Pub Date : 2020-08-29 , DOI: 10.1016/j.cherd.2020.08.023 Wei Chen , Qian Tang , Zhujun Liu , Fei Luo , Yi Liao , Shuang Zhao , Ke Zhang , Lin Cheng , Dandan Ma
|
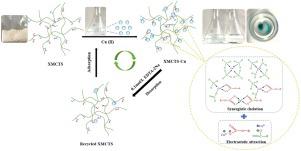
To improve the xanthogenic acidification efficiency of chitosan (CTS), a novel chitosan-based adsorbent (XMCTS) with strong chelating ability was prepared by using maleic anhydride as the connecting bridge for the first time. Some characterization methods such that scanning electron microscope, nuclear magnetic resonance spectroscopy, X-ray photoelectron spectroscopy, and thermogravimetric were employed to analysis the structure of copolymers, and verify the successful synthesis of XMCTS. Comparative study found that the introduction of maleic anhydride increased the graft rate and acid stability of XMCTS. Impact of dosage, pH, contact time, initial concentration, and temperature on adsorption performance were explored. Compared with CTS, XMCTS showed better adsorption performance, reaching a maximum of 201.43 mg/g at 40 °C. The adsorption kinetics of XMCTS fitted well with the pseudo-second-order model, and the adsorption isotherm was well described by the Langmuir isotherm model. Adsorption thermodynamics indicated that the adsorption of Cu(II) was a spontaneous endothermic process. The analysis of adsorption mechanism indicated that the strong synergistic chelation and electrostatic attraction of the amino, carboxyl, hydroxyl and xanthate groups contributed to the adsorption of Cu(II) effectively.
中文翻译:

制备具有多功能协同作用的新型脱乙酰壳多糖吸附剂,以去除Cu(II):马来酸酐为连接桥
为了提高壳聚糖(CTS)的黄原酸化效率,首次以马来酸酐为连接桥,制备了一种具有较强螯合能力的新型壳聚糖基吸附剂(XMCTS)。利用扫描电子显微镜,核磁共振波谱,X射线光电子能谱和热重分析等表征方法对共聚物的结构进行了分析,验证了XMCTS的成功合成。比较研究发现,引入马来酸酐可提高XMCTS的接枝率和酸稳定性。探索了剂量,pH,接触时间,初始浓度和温度对吸附性能的影响。与CTS相比,XMCTS表现出更好的吸附性能,在40°C时最高达到201.43 mg / g。XMCTS的吸附动力学很好地拟合拟二阶模型,吸附等温线可以用Langmuir等温线模型很好地描述。吸附热力学表明,Cu(II)的吸附是自发的吸热过程。吸附机理的分析表明,氨基,羧基,羟基和黄原酸酯基团的强协同螯合和静电吸引作用有效地吸附了Cu(II)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号