当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activity and specificity studies of the new thermostable esterase EstDZ2.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-08-29 , DOI: 10.1016/j.bioorg.2020.104214 Kamela Myrtollari 1 , Nikolaos Katsoulakis 1 , Dimitra Zarafeta 2 , Ioannis V Pavlidis 1 , Georgios Skretas 2 , Ioulia Smonou 1
中文翻译:

新型热稳定酯酶EstDZ2的活性和特异性研究。
更新日期:2020-09-11
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-08-29 , DOI: 10.1016/j.bioorg.2020.104214 Kamela Myrtollari 1 , Nikolaos Katsoulakis 1 , Dimitra Zarafeta 2 , Ioannis V Pavlidis 1 , Georgios Skretas 2 , Ioulia Smonou 1
Affiliation
|
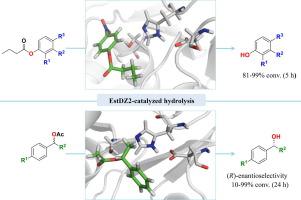
In this paper, we study the activity and specificity of EstDZ2, a new thermostable carboxyl esterase of unknown function, which was isolated from a metagenome library from a Russian hot spring. The biocatalytic reaction employing EstDZ2 proved to be an efficient method for the hydrolysis of aryl p-, o- or m-substituted esters of butyric acid and esters of secondary alcohols. Docking studies revealed structural features of the enzyme that led to activity differences among the different substrates.
中文翻译:

新型热稳定酯酶EstDZ2的活性和特异性研究。
在本文中,我们研究了功能未知的新型热稳定羧基酯酶EstDZ2的活性和特异性,该酶是从俄罗斯温泉的一个基因组库中分离出来的。采用EstDZ2生物催化反应被证明是芳基的水解的有效方法p - ,Ö -或米取代丁酸的酯和仲醇的酯。对接研究揭示了导致不同底物活性差异的酶的结构特征。









































 京公网安备 11010802027423号
京公网安备 11010802027423号