当前位置:
X-MOL 学术
›
Arab. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficiency of Acacia Gummifera powder as biosorbent for simultaneous decontamination of water polluted with metals
Arabian Journal of Chemistry ( IF 5.3 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.arabjc.2020.08.022 Bassem Jamoussi , Radhouane Chakroun , Cherif Jablaoui , Larbi Rhazi
Arabian Journal of Chemistry ( IF 5.3 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.arabjc.2020.08.022 Bassem Jamoussi , Radhouane Chakroun , Cherif Jablaoui , Larbi Rhazi
|
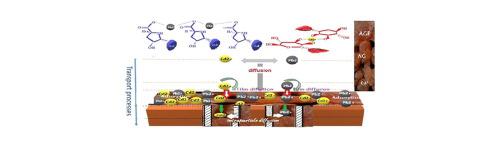
Abstract Biosorbent materials represent an interesting alternative to classic methods of metal removal from industrial effluents. Acacia biomass showed a higher absorption capacity for heavy metals than living biomass. This study aimed to evaluate the bioadsorption of Lead and Cadmium onto Acacia Gummifera gum, using batch experiment. The structural characterization of the biosorbent was carried out using FT-IR, SEM, BET, TGA and DSC analysis. The adsorption equilibrium was reached within 15 min. A maximum uptake of 18.3 mg.g−1 Pb2+ and 9.57 mg.g−1 Cd2+ was achieved at pH 6.5. The metal ions seemed to be removed exclusively by ion exchange, physical sorption and chelation. The biomass of A. Gummifera powder was found to be effective for lead and cadmium removal with respectively 97% and 86% sorption efficiency at a concentration of 100 mg/L, in aqueous media. Parameters affecting adsorption capacities such as biosorbent dosage, initial metal concentration, temperature, and pH are discussed in detail. Furthermore, adsorption thermodynamics, kinetics, and equilibrium were studied and fitted by different models. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms were used to compare adsorption data at equilibrium. The adsorption kinetics data were found to be best fitted by the pseudo-second-order model, and the adsorption isotherm was well fitted with the Langmuir model. The calculated thermodynamic parameters (ΔG0, ΔS0 and ΔH0) indicated a spontaneous and exothermic biosorption of both metal ions onto Acacia Gummifera. Moreover, chromatograms obtained by size exclusion chromatography coupled with multi-angle laser light scattering detection system (SECMALLS) showed the formation of complexes between the arabinogalactan-peptide (AGP) and glycoprotein (GP) Acacia moieties and the two studied metal ions. The analysis of the FTIR spectra of dried Acacia and that of Acacia loaded with lead and cadmium in aqueous media suggests that the surface functional groups such as amides and carboxy groups might be involved in the metal removal process. The extent of adsorption for both metals increased along with an increase of the A. Gummifera biomass dosage. A. Gummifera biomass, which is safe, of low-cost, and highly selective, seems therefore to be a promising substrate for simultaneous trapping of Pd and Cd ions in aqueous solutions.
中文翻译:

金合欢粉作为生物吸附剂同时净化受金属污染的水的效率
摘要 生物吸附材料代表了从工业废水中去除金属的经典方法的一种有趣的替代方法。金合欢生物质对重金属的吸收能力高于活生物质。本研究旨在通过批量实验评估铅和镉在金合欢树胶上的生物吸附。使用 FT-IR、SEM、BET、TGA 和 DSC 分析对生物吸附剂进行了结构表征。在 15 分钟内达到吸附平衡。在 pH 6.5 时,最大吸收量为 18.3 mg.g-1 Pb2+ 和 9.57 mg.g-1 Cd2+。金属离子似乎只能通过离子交换、物理吸附和螯合去除。发现 A. Gummifera 粉末的生物量对铅和镉的去除有效,在 100 mg/L 的浓度下分别具有 97% 和 86% 的吸附效率,在水介质中。详细讨论了影响吸附能力的参数,例如生物吸附剂剂量、初始金属浓度、温度和 pH 值。此外,还研究了吸附热力学、动力学和平衡,并通过不同的模型进行了拟合。Langmuir、Freundlich、Temkin 和 Dubinin-Radushkevich 等温线用于比较平衡时的吸附数据。发现吸附动力学数据最适合拟二级模型,吸附等温线与朗缪尔模型拟合良好。计算出的热力学参数(ΔG0、ΔS0 和 ΔH0)表明两种金属离子在 Acacia Gummifera 上的自发和放热生物吸附。而且,通过尺寸排阻色谱结合多角度激光散射检测系统 (SECMALLS) 获得的色谱图显示,阿拉伯半乳聚糖肽 (AGP) 和糖蛋白 (GP) 金合欢部分与两种研究的金属离子之间形成了复合物。干燥金合欢的 FTIR 光谱分析和在水性介质中负载铅和镉的金合欢的 FTIR 光谱分析表明,表面官能团如酰胺和羧基可能参与金属去除过程。两种金属的吸附程度随着 A. Gummifera 生物质剂量的增加而增加。A. Gummifera 生物质安全、低成本和高选择性,因此似乎是在水溶液中同时捕获 Pd 和 Cd 离子的有前途的底物。
更新日期:2020-10-01
中文翻译:

金合欢粉作为生物吸附剂同时净化受金属污染的水的效率
摘要 生物吸附材料代表了从工业废水中去除金属的经典方法的一种有趣的替代方法。金合欢生物质对重金属的吸收能力高于活生物质。本研究旨在通过批量实验评估铅和镉在金合欢树胶上的生物吸附。使用 FT-IR、SEM、BET、TGA 和 DSC 分析对生物吸附剂进行了结构表征。在 15 分钟内达到吸附平衡。在 pH 6.5 时,最大吸收量为 18.3 mg.g-1 Pb2+ 和 9.57 mg.g-1 Cd2+。金属离子似乎只能通过离子交换、物理吸附和螯合去除。发现 A. Gummifera 粉末的生物量对铅和镉的去除有效,在 100 mg/L 的浓度下分别具有 97% 和 86% 的吸附效率,在水介质中。详细讨论了影响吸附能力的参数,例如生物吸附剂剂量、初始金属浓度、温度和 pH 值。此外,还研究了吸附热力学、动力学和平衡,并通过不同的模型进行了拟合。Langmuir、Freundlich、Temkin 和 Dubinin-Radushkevich 等温线用于比较平衡时的吸附数据。发现吸附动力学数据最适合拟二级模型,吸附等温线与朗缪尔模型拟合良好。计算出的热力学参数(ΔG0、ΔS0 和 ΔH0)表明两种金属离子在 Acacia Gummifera 上的自发和放热生物吸附。而且,通过尺寸排阻色谱结合多角度激光散射检测系统 (SECMALLS) 获得的色谱图显示,阿拉伯半乳聚糖肽 (AGP) 和糖蛋白 (GP) 金合欢部分与两种研究的金属离子之间形成了复合物。干燥金合欢的 FTIR 光谱分析和在水性介质中负载铅和镉的金合欢的 FTIR 光谱分析表明,表面官能团如酰胺和羧基可能参与金属去除过程。两种金属的吸附程度随着 A. Gummifera 生物质剂量的增加而增加。A. Gummifera 生物质安全、低成本和高选择性,因此似乎是在水溶液中同时捕获 Pd 和 Cd 离子的有前途的底物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号