当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing the enthalpic contribution of hydrogen bonds by solvent shielding
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2020-08-28 , DOI: 10.1039/d0cb00108b Jonathan Cramer 1 , Xiaohua Jiang 1 , Wojciech Schönemann 1 , Marleen Silbermann 1 , Pascal Zihlmann 1 , Stefan Siegrist 1 , Brigitte Fiege 1 , Roman Peter Jakob 2 , Said Rabbani 1 , Timm Maier 2 , Beat Ernst 1
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2020-08-28 , DOI: 10.1039/d0cb00108b Jonathan Cramer 1 , Xiaohua Jiang 1 , Wojciech Schönemann 1 , Marleen Silbermann 1 , Pascal Zihlmann 1 , Stefan Siegrist 1 , Brigitte Fiege 1 , Roman Peter Jakob 2 , Said Rabbani 1 , Timm Maier 2 , Beat Ernst 1
Affiliation
|
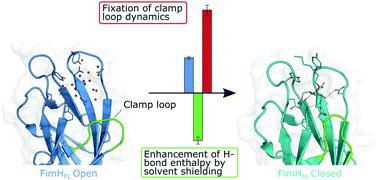
In biological systems, polar interactions are heavily burdened by high desolvation penalties resulting from strong solute–solvent interactions. As a consequence thereof, enthalpic contributions of hydrogen bonds to the free energy of binding are severely diminished. However, this effect is strongly attenuated for interactions within solvent-shielded areas of proteins. In microcalorimetric experiments, we show that the bacterial lectin FimH utilizes conformational adaptions to effectively shield its binding site from solvent. The transition into a lower dielectric environment results in an enthalpic benefit of approximately −13 kJ mol−1 for mannoside binding. However, this effect can be abrogated, if the hydrogen bond network within the binding site is disturbed by deoxygenation of the ligand. Conformational adaption leading to reduced local dielectric constants could represent a general mechanism for proteins to enable enthalpy-driven recognition of polar ligands.
中文翻译:

通过溶剂屏蔽增强氢键的热函贡献
在生物系统中,极性相互作用受到强溶质-溶剂相互作用导致的高去溶剂化惩罚的严重影响。结果,氢键对结合自由能的焓贡献严重减弱。然而,对于蛋白质溶剂屏蔽区域内的相互作用,这种效应会大大减弱。在微量热实验中,我们表明细菌凝集素 FimH 利用构象适应有效地保护其结合位点免受溶剂的影响。过渡到较低介电环境导致甘露糖苷结合的焓效益约为 -13 kJ mol -1 。然而,如果结合位点内的氢键网络受到配体脱氧的干扰,则这种效应可以被消除。导致局部介电常数降低的构象适应可以代表蛋白质实现极性配体的焓驱动识别的一般机制。
更新日期:2020-10-08
中文翻译:

通过溶剂屏蔽增强氢键的热函贡献
在生物系统中,极性相互作用受到强溶质-溶剂相互作用导致的高去溶剂化惩罚的严重影响。结果,氢键对结合自由能的焓贡献严重减弱。然而,对于蛋白质溶剂屏蔽区域内的相互作用,这种效应会大大减弱。在微量热实验中,我们表明细菌凝集素 FimH 利用构象适应有效地保护其结合位点免受溶剂的影响。过渡到较低介电环境导致甘露糖苷结合的焓效益约为 -13 kJ mol -1 。然而,如果结合位点内的氢键网络受到配体脱氧的干扰,则这种效应可以被消除。导致局部介电常数降低的构象适应可以代表蛋白质实现极性配体的焓驱动识别的一般机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号